Category: Articles


Dermatologist Talks About the ABCs of Wounds and HS Support at Annual Meeting
Mar 17, 2023 Heidi Anne Duerr, MPH Hadar Lev-Tov, MD

Health workers carry wound care packs for ‘tranq’ users
Health workers carry wound care packs for ‘tranq’ users

Nonanimal Euglena gracilis-Derived Extracellular Vesicles Enhance Skin-Regenerative Wound Healing
Yuri Ko, Hwira Baek, Jee-Hyun Hwang, Youngseok Kim, Kyung-Min Lim, Junoh Kim, Jin Woong Kim




Fish Skin Grafts Versus Alternative Wound Dressings in Wound Care: A Systematic Review of the Literature
Mohamed Ibrahim • Haneen S. Ayyoubi • Layth A. Alkhairi • Hozaifa Tabbaa • Isaac Elkins • Ravish Narvel
Intermittent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance
Masaru Usui, Yutaka Yoshii, Stanislas Thiriet-Rupert, Jean-Marc Ghigo & Christophe Beloin
Study Finds Vomaris Bioelectric Technology Significantly Decreased Biofilm in Burn Patients
Sumit Puri, CIO, Max Healthcare Scott Arnold, Executive Vice President & CIO, Tampa General Hospital Georgia Mitchell, Director, Clinical and Medical Affairs, Stryker (Spine Division)
Antibacterial, Anti-Biofilm and Pro-Migratory Effects of Double Layered Hydrogels Packaged
with Lactoferrin-DsiRNA-Silver Nanoparticles for Chronic Wound Therapy Centre for Drug Delivery Technology, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur 50300, Malaysia

Weight-bearing physical activity in people with diabetes-related foot disease: A systematic review
Jaap Van Netten, Vera Fijen and and Sicco Bus.



Effect of ultrasound-supported wound debridement in subjects with diabetic foot ulcers: A meta-analysis
Haiting Chen, Ting Xiao, Ling Zhang, Ning Liu, Xia Liang, Tuodi Li, Jinying Wang, Yaozhong Peng, Yanping Liu, Jiali Xu

How to Make Dakin’s Solution
Many have wanted instructions for making the solution developed by the British Chemist Henry Dakin and the French Surgeon Alexis Carrel. To that end, we enclose the recipe.



REGENATIVE LABS ANNOUNCES PUBLICATION OF GROUNDBREAKING PAPER ON
WHARTON’S JELLY APPLICATIONS, OPENING THE DOOR FOR EXPANDED TREATMENT OF THE SI JOINT Dr. Albert Lai

Factors associated with severity and anatomical distribution of diabetic foot ulcer in Uganda:
a multicenter cross-sectional study Bienfait Mumbere Vahwere, Robinson Ssebuufu, Alice Namatovu, Patrick Kyamanywa, Ibrahim Ntulume, Isaac Mugwano, Theophilus Pius, Franck Katembo Sikakulya, Okedi Francis Xaviour, Yusuf Mulumba, Soria Jorge, Gidio Agaba & George William Nasinyama

Pain Management With Topical Ibuprofen in Partial-Thickness Burn Wounds and
Effects on Wound Healing: A Prospective Randomized Clinical Study Ali Emre Akgun, MD Merve Alkin, MD

Addressing the Variety and Complexity of Wounds through Innovations in Negative Pressure Wound Therapy
Amanda Estapa, ACNP-BC, CWS, FACCWS, DAPWCA Vice President of Clinical Services MedCentris Hammond, LA Dot M. Weir, RN, CWON, CWS Clinician Saratoga Hospital Wound Healing Gansevoort, New York Colin J. Traynor, DPM, CWSP Adjunct Assistant Professor Samuel Merritt University ORINDA, CA

Eliminating Bias in Clinical Trials: Endpoints, Interpretation, and Presentation with Dr. Suzanne Bakewell
Dr. Suzanne Bakewell, Chief Scientific Officer for skin care company Omeza

The Role of Personal-use Negative Pressure Wound Therapy with Enhanced Functionality
in Achieving Wound-related Treatment Goals: A Small Prospective Study Robert F. Mullins Joan Wilson Zaheed Hassan Bounthavy Homsombath Beretta Craft-Coffman Rey Paglinawan Inez Cregan Shawn Fagan

Wound Biomarkers: Measuring What We Manage
Windy Cole, DPM, CWSP

Unique Insights from Experts: Mechanisms for Managing Diverse and Challenging Wound Etiologies with Instillation Therapy
Elizabeth Faust, MSN, CRNP, CSWS, CWOCN-AP, DAPWCA CRNP, WOCN Tower Health System Leesport, PA Paul J Kim, DPM, MS, FACFAS, FAPWH Professor, Medical Director University of Texas Southwestern Dallas, TX

Diabetic Wound Care (video)
Wound Problem After Below Knee Amputation Treated with new age creams (boric acid and Hypericum perforatum ( Sarı kantaron ), Calendula officinalis , Neem oil ( Azadirachta indica ) and Centellin.


American College of Surgeons (ACS) Clinical Congress OCTOBER 22-26, 2023 (BOSTON, MA)
conferences and events

Circumferential Leg Ulcers With NPWT
Sandra Wainwright, MD

The Role of the Plastic Surgeon in Wound Care
Richard Simman Fuad-Tahsin Abbas Darren Gordon

Preliminary evaluation of dual-energy CT to quantitatively assess bone marrow edema
in patients with diabetic foot ulcers and suspected osteomyelitis

An Algorithmic Approach to Negative Pressure Wound Therapy: Home Healthcare and Beyond
FACULTY Todd Shaffett, DNP, FNP, CWS, FACCWS, DAPWCA President MedCentris Hammond, LA Dot Weir, RN, CWON, CWS Clinician Saratoga Hospital Wound Healing Gansevoort, NY

Primary Cutaneous Mucormycosis of the Lower Extremity in a Male Patient With Diabetes
Nicholas Chang James McKee Valerie Marmolejo Arnold Paul C. Cua

Recommendations for Wounds After Flaps and Grafts
Michael Desvigne, MD, FACS, CWS, FACCWS Dr. Desvigne will present practical examples and case studies regarding the treatment of flaps and grafts after procedures, such as metatarsal amputation and the treatment of non-healing wounds. Dr. Desvigne will also discuss tools and methods he has used in the treatment of these amputation sites and non-healing wounds.

Addressing Both the What and the Why of Clinical Documentation
by Caroline Fife, M.D.

Managing the Landscape of Wound Types Working from a Comprehensive Algorithm
Vickie R. Driver, DPM, MS, FACFAS System Wide Medical Director INOVA Wound Healing and Hyperbaric Centers Falls Church Virginia Barry University , Miami Shores Florida Anderson Island, WA Daniel L. Kapp, MD Plastic Surgeon Daniel Kapp MD Plastic Surgery and Wound Care West Palm Beach, Florida Andrew J. Rader, DPM, FAENS, FACFAOM, FASPS, FAPWCA, FACCWS…

Early Skin Temperature Characteristics of the Kennedy Lesion (Kennedy Terminal Ulcer)
Karen Lou Kennedy-Evans, RN, FNP, APRN-BC; Deanna Vargo, BSN, RN, CWS, FACCWS, CWOCN; Leslie Ritter, PhD, RN; Diane Adams, BSN, RN, CWCN; Suzanne Koerner, BSN, RN, CWOCN; Ellen Duell, APRN, CWOCN, ACNS-BC

Preventing Mortality in Patients with DFUs
BY LEONARD A. LEVY, DPM, MPH

Eugene artist uses ‘good, gross art’ to illustrate ailments worsened by homelessness
Anyone can get the wounds depicted, but a lack of access to hygiene can make them so much more likely. Tatiana Parafiniuk-Talesnick


From the labs. Biofilm buster
Nanocomposite coating inhibits biofilm formation during post-operative care



Fragile Feet and Trivial Trauma: Communicating the Etiology of Diabetic Foot Ulcers to Patients
Gustav Jarl, Jaap van Netten, and Peter Lazzarini with another important contribution

Uncommon Wounds: Frostbite and Winter Woes
By Lauren Lazarevski, RN, BSN, CWOCN

Wound infections: an overview
Martha Williams
Responses to “Who Should Assess and Stage Pressure Injuries in Hospitalized Patients?”
Michael A. Bain, Garrett Wirth, Robert X Murphy
Wound swab quality grading is dependent on Gram smear screening approach
Shawn T. Clark, Jessica D. Forbes & Larissa M. Matukas

Design, development, in-vitro and in-vivo evaluation of polylactic acid-based multifunctional
nanofibrous patches for efficient healing of diabetic wounds Isra H. Ali, Islam A. Khalil Ibrahim M. El-Sherbiny







Risk factors for wound healing complications after revascularization for MMD with complete Y-shaped incision
Chenchao Wang, Hongwei Li, Yang Dong, Hao Wang, Dongpeng Li, Chengbin Zhao, Lei Cao, Kaiwen Sun, Jiefeng Geng & Bo Yang

Diabetic Foot: Facts and Figures from DF Blog
David Armstrong
Congress Takes a Positive Swing at an Unsung Public Health Crisis: Preventing Pressure Injuries in Veterans Affairs Facilities
Padula, William V. PhD; Black, Joyce M. PhD, RN; Garcia, Aimee MD; Mishra, Manish K. MD, MPH
REGENATIVE LABS AND BROTHERS IN ARMS FOUNDATION ANNOUNCE NEW
PARTNERSHIP TO OFFER STATE OF THE ART CARE TO INJURED VETERANS WITH 5 MILLION DOLLAR DONATION
Both organizations call on physicians to assist Veterans.
PENSACOLA, Fla. , Feb. 27, 2023 /PRNewswire/ — Regenative Labs (Regenative), a leading HCT/P manufacturer, is partnering with Brothers in Arms Foundation (BIAF), a 501©3 nonprofit providing logistical and financial support to wounded, ill, injured and fallen Marines, sailors, and the families of those who served. Together, both organizations are dedicated to giving back and improving the quality of life for those who have served our country.
Regenative will donate approximately $5 million dollars of its state-of-the-art Wharton’s Jelly product, ProText™, to BIAF which will facilitate the care of qualifying veterans. To bring this care to veterans, Regenative and BIAF need the help of physicians to apply the product.
Physicians across the country may join in supporting our veterans by making a qualified donation of their time under the Brothers in Arms Foundation 501©3 non-profit.
“We’re extremely proud of this partnership,” shared Regenative Labs CEO, Tyler Barrett. “Ultimately our products are about improving patient quality of life. My grandfather served in the Marines in Korea, and so being able to serve those like him in unison with the mission of Brothers in Arms is important to me,” Barrett concluded.
Regenative’s Wharton’s Jelly product is a structural connective tissue allograft intended for homologous use to replace or supplement missing or damaged tissue directly at the site of a structural defect. Some doctors have used the product for muscle and cartilage tears and to replace missing or damaged tissue due to wounds and tissue defects, instead of masking the underlying connective tissue issue.
Regenative has been tracking patient outcomes of applications of their product for over two years. Retrospectively, the results have shown improvements greater than 33 percent in patient reported WOMAC improvement.
It is Regenative’s and BIAF’s goal to provide veterans with the best quality of life possible.
“We’re committed to bettering the quality of life for our veterans,” said Brothers in Arms Foundation President, Phillip Noblin. “This new, innovative treatment will give them the hope and support needed to heal. The partnership with Regenative Labs will further our mission of serving those who’ve served us,” said Noblin.
Physicians interested in taking care of qualified veterans by donating their services through a 501©3 non-profit may visit: https://regenativelabs.com/biaf-physician-time-and-services-donation/
Veterans who wish to learn more, or to refer their physician today may visit: https://regenativelabs.com/biaf/
One can make a monetary donation through the BIAF.
About Regenative Labs: Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with daily challenges of innovations in healthcare, the company provides non-addictive, non-invasive options for patients. Regenative Labs’ expert product research and development team complies with FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines. Learn more at Regenative’s website: www.regenativelabs.com
About Brothers in Arms Foundation: Brothers in Arms Foundation is a 501c3 non-profit founded in 2009 that exists to provide financial and logistical support to wounded, ill, injured and fallen Marines, sailors, and the families of those who served within the Marines Special Operations community. Over the years, the foundation has supported active-duty Marines, sailors, veterans and military families with programs and services ranging from financial assistance, funding vocational and quality of life initiatives, as well as funeral support and childcare. Learn more at:
https://www.brothersinarmsfoundation.org
SOURCE Regenative Labs
Foot ulcers in systemic sclerosis escape surgical amputation
Dr Giuseppina Abignano

Albuminuric diabetic kidney disease increases risk for foot ulcers
Vincent Rigalleau

New paper targets prevention of foot and lower limb diseases
Dr. Peter Roberts


Chronic Venous Insufficiency and Lymphedema With Papillomatosis Cutis Lymphostatica,
Hyperkeratosis, and Skin Ulcers: A Case Report Jayani Senanayake • Sanket Chaudhari • Rangin Haji Rahman • Sally Madanat • Frederick Tiesenga


Roundtable Discussion from The American College of Clinical Wound Specialists (ACCWS)
Frank Aviles, PT, CWS, FACCWS, CLT-LANA, ALM, AWCC, DAPWCA


Marching Forward With Global Pressure Injury Data
Elizabeth A. Ayello, R. Gary Sibbald



Bilayer hydrogel dressing with lysozyme-enhanced photothermal therapy for biofilm eradication
and accelerated chronic wound repair biofilm eradication and accelerated chronic wound repair Yizhen Wang, Qijun Lv, You Chen, Langtao Xu, Miao Feng b, Zhiyong Xiong, Jiajun Li, Jie Ren, Jie Liu, Bo Liu
Long-term Follow-up in Restoring Soft Tissue Deficits with A Novel Human Adipose Allograft Matrix*
Speaker: Matthew Regulski, DPM FFPM RCPS (Glasgow)

Wound Masterclass | Emerging Technologies in Clinical Practice
Yuanwen Jiang, Artem Trotsyuk, Simiao Niu, Dominic Henn, Kellen Chen, Chien-Chung Shih, Madelyn R. Larson, Alana M. Mermin-Bunnell, Smiti Mittal, Jian-Cheng Lai, Aref Saberi, Ethan Beard, Serena Jing, Donglai Zhong, Sydney R. Steele, Kefan Sun, Tanish Jain, Eric Zhao, Christopher R. Neimeth, Willian G. Viana, Jing Tang, Dharshan Sivaraj, Jagannath Padmanabhan, Melanie Rodrigues, David P.…

First patients enrolled in trial of RLF-TD011 wound spray for EB
by Patricia Inácio, PhD | February 17, 2023

Cicatrization of Oncological Wounds by Autologous Fibrin Biopolymer Dressing Treatment: A Case Series
Chayane K.L. de Carvalho Beatriz Luci Fernandes Michel M. Dalmedico Mauren Abreu de Souza

VASCULAR Scientific Sessions
Presented by the Society for Vascular Medicine September 7 — September 10, 2023 Grand Hyatt • Washington, DC conferences and events

The Amputee Coalition 2023 National Conference
Renaissance Orlando at SeaWorld®, August 2 -5, 2023 conferences and events

Skin Substitute Reimbursement and Coding Best Practices Webinar Series
Dr. Jeffrey Lehrman, DPM, FASPS, MAPWCA, CPC, CPMA Terrence Mabry – MIMEDX Regional Reimbursement Director

ADVANCED WOUND CARE SUMMIT | 12-13 JULY, 2023
BOSTON, USA conferences and events

HOSPITALS LOOK TO IMPROVE WOUND CARE WITH SOFTWARE
BY ERIC WICKLUND | OCTOBER 07, 2022

Wild on Wounds – September 13-16 Hollywood Florida
conferences and events

Worldwide Research Trends on Diabetic Foot Ulcers
(2004-2020): Suggestions for Researchers Pin Deng, Hongshuo Shi, Xuyue Pan, Huan Liang, Shulong Wang, Junde Wu, Wei Zhang, Fasen Huang, Xiaojie Sun, Hanjie Zhu, Zhaojun Chen

Speaker offers pearls in epidermolysis bullosa therapies
Rebecca L. Forand

Severe wound infection by MRCNS following bilateral inguinal herniorrhaphy
Yao Du, Song Han, Yue Zhou, Hai Feng Chen, Yao Liang Lu, Zhi Yuan Kong & Wei Ping Li


Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia
Justin Chin-Bong Choi, Jorge Miranda, Neal R Barshes

Accuracy of the pedal acceleration time to diagnose limb ischemia in patients with and without diabetes using the WIfI classification
Guilherme de Castro-Santos, Patric Emerson Oliveira Gonçalves, and Túlio Pinho Navarro

Cigarette smoking and mitochondrial dysfunction in peripheral artery disease
Michelle Guo, Mary M McDermott, Philip Greenland

Observed impact of skin substitutes in lower extremity diabetic ulcers:
lessons from the Medicare Database (2015–2018) | Published Online:14 Jul 2021

VENOUS 2023 | AMERICAN VENOUS FORUM
February 22-25 San Antonio TX conferences and events

A Real-world Study of a Calcium Alginate Dressing for Various Wound Etiologies on Clinical Performance, Usability, and Safety
Renata Bienias Marco Feulner Stefan Hergenröder Hannes Schrammel Lisa Spreitzer Elzbieta Szkile Adam Zielinski Claas Roes

A Multi-Center, Single-Blinded Randomized Controlled Clinical Trial Evaluating the Effect of
Resorbable Glass Fiber Matrix in the Treatment of Diabetic Foot Ulcers David G. Armstrong, DPM, MD, PhD

Creating a Wound Care Culture
David Navazio 2/7/2023

DFCon 2023 – – DIABETIC FOOT CONFERENCE
28th – 30th of September, 2023 – Anaheim, California conferences and events

Minimizing Bias in a Diabetic Foot Ulcer Clinical Evaluation: Analysis of the HIFLO Trial
Paul Glat Lisa Gould Lael J Pickett Douglas M Arm Clinical Trial Clinical Research Wound Healing Diabetic Foot Ulcer Clinical Trial Bias Minimizing Bias
Chronic Inhibitory Bacterial Load (CIBL): New Clinical Terminology for Elevated Levels of Bacteria in Wounds that Preclude Healing
Findings from a New Clinical Study Using MolecuLight Imaging of Diabetic Foot Ulcers Prompts New Diagnostic Terminology Enabling Proactive Infection Management
TORONTO, Feb. 14, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated bacterial loads in and around wounds, announced the publication of “Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers“1 in International Wound Journal. The publication reports on the analysis of 138 diabetic foot ulcer wounds, highlighting the frequent presence of healing delays and elevated bacterial burden as identified through standard clinical assessment, fluorescence imaging (MolecuLight i:X®), and quantitative microbiology.
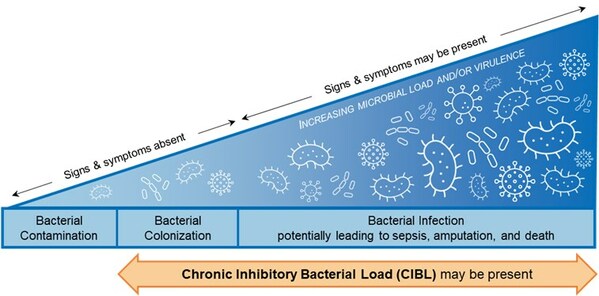
Chronic inhibitory bacterial load (“CIBL”) on the bacterial-infection continuum. Based on the International Wound Infection Institute (IWII) 2022 wound infection continuum (CNW Group/MolecuLight)
The emergence of data on the link between bacterial load and healing over the last decade, together with this current study, prompted study authors David G. Armstrong, Michael E. Edmonds, and Thomas E. Serena to define new clinical terminology, chronic inhibitory bacterial load (CIBL). CIBL is defined as “the chronic presence of bacterial microorganisms in a wound or its surrounding tissue at loads which can damage tissues and be inhibitory to healing, as well as require clinical intervention, with or without the presence of clinical symptoms”.
MolecuLight fluorescence imaging is currently the only way to detect and locate CIBL at the point of care. This term enables the proactive diagnosis of CIBL early along the bacterial-infection continuum, to facilitate its targeted removal, promote healing, and prevent the sequelae of infection in frequently asymptomatic diabetic ulcers.
Key findings of the study include:
- Less than 12% of diabetic ulcers exhibited clinical symptoms of bacteria and infection, despite the presence of loads >104 CFU/g in over 90% (average bacterial load of 108 CFU/g). Even as bacterial loads increased up to >108 CFU/g, detection of clinical signs and symptoms of infection did not increase.
- Bacterial loads >104 CFU/g can preclude wounds from healing through various biological mechanisms and are contraindicated for many advanced therapies. This study showed that the occurrence of delayed healing increased alongside bacterial load.
- Fluorescence imaging using MolecuLight increased sensitivity for the detection of CIBL across loads 104–109 (p < .0001), peaking at 92.6% for bacterial loads >108 CFU/g. This was 8.3 times superior to standard clinical assessment alone.
- Fluorescence imaging further showed that 84.2% of ulcers contained high loads in the periwound region, an area that is frequently overlooked.
Infection prevention is a key goal of CIBL’s introduction, adoption, and management. CIBL is the result of these seasoned wound care clinicians’ long-time advocacy for proactive wound management as they see firsthand the devastating consequences of delayed treatment. “Infection is the greatest destroyer of the diabetic foot. It is the final common pathway for most amputations, and we need to fight it as early as possible in its natural history”, says Dr. Michael E. Edmonds, one of the paper’s authors and Consultant of Diabetologist at the Diabetic Foot Clinic, King’s College Hospital Foundation Trust in London, UK. “CIBL localization and proactive management is a crucial strategy in reducing unnecessary amputations and saving lives”, he concludes.
As MolecuLight is the only device capable of detecting elevated bacterial loads in wounds in real-time, regions of CIBL can be non-invasively and accurately detected and mapped. The device provides clinicians with immediate feedback to guide their therapeutic decision-making process in a number of clinical settings from the outpatient clinic to the operating room. Multiple routine procedures are enhanced by its proven capabilities, such as debridement, wound hygiene, and preparation for advanced therapies resulting not only in better outcomes,3,4 but more rational resource consumption and antimicrobial stewardship.4
“There is also a meaningful role for fluorescence imaging with MolecuLight in antimicrobial stewardship. This is critical considering that approximately 70% of patients with diabetic foot ulcers are prescribed antibiotics at some point during their care, and over 80% are prescribed antimicrobial dressings3, often in a haphazard manner”, says Dr. Thomas Serena, study author and the Founder and Medical Director of The SerenaGroup®. “Diagnostic uncertainty has been listed as a key factor in antibiotic overuse in wound care. Fluorescence signals as a real-time imaging biomarker of CIBL could enable clinicians to more effectively leverage hygiene-based strategies to remove bacteria rather than resorting to antibiotics”.
“The definition of an infection’s genesis and its resolution is a clinical one”, notes Dr. David G. Armstrong, study author, Professor of Surgery at the University of Southern California, and founder and co-Director of the Southwestern Academic Limb Salvage Alliance (SALSA). “The problem is that many objective local signs may be blunted in the chronic wound and it is likely that we are not yet effectively measuring what we manage. Fluorescence imaging of chronic inhibitory bacterial load (CIBL) is positioned to potentially change contemporary paradigms of wound management. We are hopeful that this new clinical term, CIBL, can be a key indicator to enable pre-infection intervention such as debridement or modification of wound therapy.”
The MolecuLight i:X and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 65 peer-reviewed publications involving 1,500 patients, they are used by leading wound care facilities globally.
References
1 Armstrong DG, Edmonds ME, Serena TE. Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers. Int Wound J. 2023;20(2):554-566
2 Wounds International (2022) International Consensus Update 2022 International Wound Infection Institute (IWII) Wound Infection in Clinical Practice: Principles of best practice. Available from https://woundinfection-institute.com/
3 Price N. Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: retrospective analysis of 229 foot ulcers. Diagnostics (Basel). 2020;10(11):927.
4 Rahma S, Woods J, Nixon JE, Brown S, Russell DA. The use of point-of-care bacterial autofluorescence imaging in the Management of Diabetic Foot Ulcers: a pilot randomised controlled trial. Diabetes Care. 2022;45:1601-1609.
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight ‘s suite of commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
SOURCE MolecuLight

.
Where: San Diego Convention Center, San Diego, California, USA When: Friday, June 23, 2023 (beginning at 10:30 a.m.) through Monday, June 26, 2023 (concluding at 4:15 p.m.) conferences and events

APMA 2023 Annual Scientific Meeting (The National)
DATE July 13 – 16PLACE Nashville, TN conferences and events



Chlorhexidine-impregnated Tulle Gras Versus Polyethylene Nonadhesive Film for Dressing of
Donor Site Wounds in Cases of Split-thickness Skin Graft Iyer Sandhya P Prabhakar Subramaniyan Gade Sujata V

Wounds Canada 2023 Conference
September 28-30, 2023

Recommendations for Preventing Partial First Ray Amputation Failure
Samuel Adegboyega, DPM

EWMA 2023 in Milan
3-5 May 2023 in Milan, Italy Sebastian Probst, Luc Teot, Laura Stefanon conferences and events


How Innovation in Wound Management is Advancing Care
Jeanine Maguire, Vice President, Skin Health & Wound Care Integrations, Genesis


Home-Based Wound Care: Insights on Challenges and Rewards
Laura Kaiser, RN, MSN, APRN-BC, CNS, WOCN

WCPC Wound Certification Prep Course
Raleigh, NC, March 11–12, 2023

The SoTV 2023 Annual Conference
the Kingsgate Conference Centre, 26 – 27 April conferences and events


Efficacy of Placental and Umbilical Tissue in an Infected Diabetic Foot Ulcer
Harry Schneider, DPM, FACFAS

NPIAP 2023 Annual Conference – March 17
Marriott Marquis San Diego Marina 333 W Harbor Drive San Diego, California 92101 conferences and events

WoundCon Spring 2023 – March 10, 2023
Based on the latest evidence and innovations in wound care, WoundCon Spring 2023 offers practical strategies that you can immediately implement into your practice. Featuring free registration for licensed healthcare professionals and the convenience of interactive education with world-renowned specialists on March 10, this a must-attend CME/CNE meeting. conferences and events

Wound Healing and Critical Care – February 23-24, 2023 Dubai
Future Advancements and Strategies in Wound Healing and Critical Care conferences and events

2023 Wound Care Symposium – March 16, 2023
IEA Professional Development Center 3440 Liberty Drive Springfield, IL 62704 conferences and events

WOUND CARE TODAY 2023 – March 1, 2023
MARSHALL ARENA MILTON KEYNES conferences and events

2023 Symposium on Advanced Wound Care (SAWC) & Wound Healing Society (WHS) Annual Meeting
In-Person Event • Apr 26 – 29, 2023 • National Harbor, Maryland, USA conferences and events
Mayo Clinic Wound Symposium March 1, 2023
The Edges of Wound Care 2023 Karen L. Andrews, M.D. Jennifer L. Blanco, M.S.N., R.N., CNOR Brianna M. Skrukrud, APRN, C.N.P. Danielle T. Vlazny, P.A.-C., M.S. Saranya P. Wyles, M.D., Ph.D. conferences and events

WOCNEXT® 2023 June 4-7, 2023
conferences and events


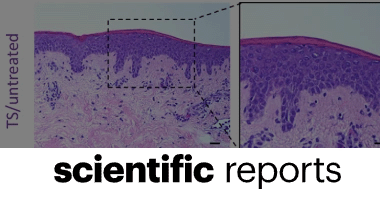
Anti-biofilm effects and healing promotion by silver oxynitrate-based dressings
Christopher Doherty, Charlotte V. Byrne, Sajwa Baqader, Cecile El-Chami, Andrew J. McBain & Helen A. Thomason

What’s Trending in Wound Care, Edition 1
Wed, Feb 8, 2023 2:00 PM – 3:00 PM EST

B is for Better: Vitamin B12 and Diabetic Foot
Anne Lin, PharmD, BCPS

Blumenauer Introduces the Promoting Access to Diabetic Shoes Act
Earl Blumenauer
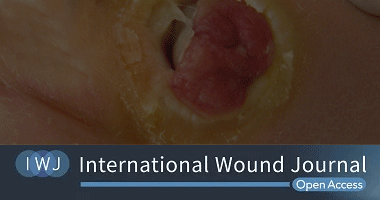
Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers
David G. Armstrong, Michael E. Edmonds, Thomas E. Serena

Journal of Wound Care Conference 2023
24TH FEBRUARY 2023 AMERICA SQUARE CONFERENCE CENTRE conferences and events


ACFAS 2023 SCIENTIFIC CONFERENCE
LOS ANGELES FEBRUARY 9-12 conferences and events


APMA ANNUAL SCIENTIFIC MEETING –
NASHVILLE JULY 13-16, 2023 conferences and events

The National Pressure Injury Advisory Panel (Inc.) Annual Conference
San Diego, California • March 17-18, 2023 conferences and events

The Biomechanical Effects of Diabetic Neuropathy
Elizabeth Ansert, MA, DPM, MBA

Applications of Bacterial Biopolymers in Biomedicine
By Michael Greenwood, M.Sc. Reviewed by Emily Henderson, B.Sc.

Diabetes Core Update – February 2023
Neil Skolnik, M.D and John J. Russell, M.D

36th Annual Lake Tahoe Ski Seminar IFAF
conferences and events

9th International Symposium on the Diabetic Foot
10 – 13 May 2023 | World Forum The Hague – The Netherlands conferences and events



Topical Morphine Gel Can Help Ease Pain of EB Wounds, Report Shows
by Marisa Wexler, MS

Allogenic Adipose-Derived Stem Cells in Diabetic Foot Ulcer Treatment
Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact Beata Mrozikiewicz-Rakowska, Ilona Szabłowska-Gadomska, Dominik Cysewski, Stefan Rudziński, Rafał Płoski, Piotr Gasperowicz, Magdalena Konarzewska, Jakub Zieliński, Mateusz Mieczkowski, Damian Sieńko, Tomasz Grzela0, Maria Noszczyk1, Barbara Paleska, Leszek Czupryniak, Malgorzata Lewandowska-Szumiel

Clinical Classification of the Diabetic Foot Syndrome Adapted to ICD-10 as a Solution
to the Problem of Diagnostics, Statistics and Standardisation Pavel Lukin, Alex G Kuchumov, Mikhail F Zarivchatskiy, Tatyana Kravtsova


Counteracting the Consequences of Diabetic Feet
from 2019 BY MARC A. BRENNER, DPM, STANLEY R. KALISH, DPM, PRADEEP ALBERT, MD, AND ALBERT RAMINFARD, DO


The Latest in Wound Healing Science
Carrie Nagorka, Senior Editor
Preventing Bedsores in Nursing Home Patients
Valerie K. Sabol, PhD, MBA, ACNP, GNP, ANEF, FAANP, FAAN
Counteracting the Consequences of Diabetic Feet
BY MARC A. BRENNER, DPM, STANLEY R. KALISH, DPM, PRADEEP ALBERT, MD, AND ALBERT RAMINFARD, DO
VIDEO: Amniotic membrane allograft offers easy placement for ulcers, abrasions
Karl G. Stonecipher, MD
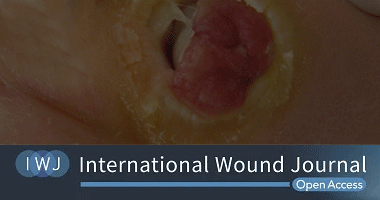
Efficacy and safety of topical oxygen therapy for diabetic foot ulcers:
An updated systematic review and meta‐analysis
Instantaneous Clearing of Biofilm (iCBiofilm): an optical approach to revisit bacterial and fungal biofilm imaging
Shinya Sugimoto & Yuki Kinjo
REGENATIVE LABS AND DR. MICHAEL LAVOR ANNOUNCE CASE STUDY DEMONSTRATING
NOVEL APPLICATION OF UMBILICAL CORD FLOWABLE TISSUE ALLOGRAFTS IN DECUBITUS ULCERS
PENSACOLA, Fla., Jan. 23, 2023 /PRNewswire/ — A case study analysis of two patients has been presented by Regenative Labs (Regenative), a leading HCT/P manufacturer, in collaboration with Dr. Michael Lavor of Saguaro Surgical. This case study demonstrates the use of Wharton’s Jelly, a connective tissue, allografts in combination with standard of care wound practices to accelerate the healing of refractory, Stage IV sacral wounds in paralyzed patients.
In reference to what he’s seen in the patient population regarding Wharton’s Jelly allografts, Lavor shared, “the patients have begged for more because it is the only thing that has helped them. I believe this is an excellent step prior to surgery, and will save hundreds of thousands of dollars.”
This case study demonstrates an application of Wharton’s Jelly allografts in late-stage sacral decubitus ulcers (SDU), also known as pressure sores, with associated tunneling in combination with standard of care. In the future, research may focus on the frequency and combination of procedural techniques that most efficiently promote granulation tissue formation and volumetric contracture of deep wounds with Wharton’s Jelly allografts.
“This, for wounds, is excellent for closing tunnels,” explained Dr. Lavor.
It was reported that for the first time in ten years, one patient experienced a highly accelerated wound closure rate, and observed volumetric reduction in the wound bed, healthy granulation tissue, and the resolution of deep tunneling. One patient achieved full one closure and epithelization.
Both patients in the presented case study had SDU classified as Stage IV with tissue loss and bone or tendon involvement. One patient had previously experienced a mid-sacral pressure sore with exposed tendon, bone, and tunneling for ten years. The other, had an ischial pressure sore with the same features that persisted for 30 months. After failing multiple conservative treatments such as wound vac placement, antibiotics, wet-to-dry dressings, and silver sulfadiazine dressings, both patients received several applications of Regenative’s Wharton’s Jelly allograft.
In both cases, after eight months of standardized wound care treatment combined with six applications of Regenative’s Wharton’s Jelly allograft, the wounds contracted by over 90% in depth, tunneling, and diameter.
Annually, thousands of individuals are affected by SDU. Treatment for these conditions is costly and far from perfect, with prices as high as an eye-watering 240,000 dollars for skin flap surgery. Inevitably, Stage II pressure ulcers can become serious if not handled swiftly. When deep, tunneling, and with both tendon and bone involvement, such as the two patients in this case study, late-stage pressure sores occur, and pose a great challenge to medical professionals.
Regenative is committed to providing patients with alternative options, and through what may be revealed in these studies, offering proven treatments to better address the root cause of their pain.
Regenative hopes to enlist physicians to take part in studies regarding uncovered uses. Physicians will have their outcomes highlighted, furthering the understanding of regenerative medicine and uncovering new applications for this groundbreaking field of medicine.
“The research at Regenative is very promising, and we’re calling on physicians across the country to engage with us and advance regenerative medicine,” shared Regenative Labs CEO, Tyler Barrett.
Contact Regenative to get your practice involved today.
About Regenative Labs: Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with the daily challenges of innovations in healthcare, the company provides non-addictive, non-invasive options for patients. Regenative Labs’ expert product research and development team comply with FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines. Learn more at Regenative’s website: www.regenativelabs.com
About Dr. Michael Lavor: Dr Michael Lavor has worked for over 28 years bringing the highest quality of medicine to his patients. He is currently based out of Arizona, and is planning to open his own practice within the first 2 quarters of 2023. In addition to his work as Assistant Medical Director at Saguaro Surgical, Dr. Lavor was a member of the Trauma Team at Tucson Medical Center where he also served as Chairman of the Department of General and Vascular Surgery. Dr. Lavor was board certified in General Surgery and was a fellow in the American College of Surgeons, past president of the Rocky Mountain Vascular Surgical Society, a Fellow in the Southwestern Surgical Congress, a member of the Tucson Surgical Society, a member of the International Society of Endovascular Surgery and the Pima County Medical Society. Lavor served in the Navy for ten years as a Navy Corpsman with the Marines; he returned to service in 2009 and served as a Commander / OIC of a wound surgical base in Afghanistan from 2012-2013. He also was a Clinical Associate Professor at the University of Arizona Medical Center Department of Surgery.
This article was originally published here
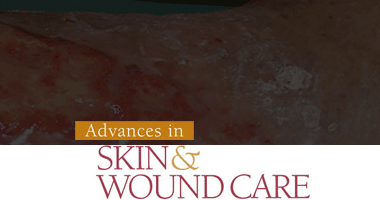
Effect of a Team Approach to Pressure Injury Management over 5 Years in a Tertiary Hospital
Suwon, Korea, Min Ji Kim, Yon Soo Jeong, Hee Joung Kim, Hyung Min Hahn, Duy Quang Thai, Jae Lee

What’s Trending in Wound Care Webinar – Edition 1
By Nancy Morgan RN, BSN, MBA, WOC

Hyperbaric Oxygen Therapy Indications Simplified
Indication #3: Radiation Injuries Denise Nemeth, MPAS, CWS Jayesh B. Shah, MD, MSc, UHM ABPM, CWSP, FAPWCA, FCCWS, FACHM FUHM, FACP

HMP Global’s SAWC Spring | WHS announces record number of wound care abstract submissions for 2023 meeting
More than 500 abstracts featuring late-breaking wound care research, new advances and techniques to improve care and outcomes for patients were submitted for poster at the 2023 event, co-located with the Diabetic Limb Salvage meeting.
HMP Global, the leading omnichannel healthcare events and education company, today announced that its 2023 Symposium on Advanced Wound Care (SAWC) Spring and Wound Healing Society (WHS) received a record-breaking number of abstract submissions for the event taking place April 26-30, strengthened by a new partnership co-locating the symposium with the Diabetic Limb Salvage conference.
Now in its 36th year, SAWC Spring | WHS is the leading meeting dedicated to the research, management, treatment, and prevention of wounds; and through the partnership with DLS, this year’s event will feature more limb salvage-focused topics on the conference agenda. The meeting is the premier multidisciplinary forum to connect practitioners, researchers, and students with the foremost experts in wound care to improve patient outcomes through education.
Symposium participants will have access to 450 posters featuring late-breaking wound care research, new advances, strategies, and techniques to improve care and outcomes for patients. More than 500 abstracts — a record number — were submitted to undergo the peer review process for poster consideration.
In addition to presenting posters in person at SAWC Spring | WHS | DLS, wound care researchers can elevate their work further by submitting abstracts for publication in the field’s preeminent, peer-reviewed journal WOUNDS, focusing on the latest advances in wound care and wound research. WOUNDS is indexed in MEDLINE/PubMED and publishes research and commentary on tissue repair and regeneration, biology and biochemistry of wound healing, and clinical management of various wound etiologies. Submission information and guidelines are available on HMP Global’s Wound Care Learning Network.
“The record-breaking number of abstract submissions this year is a testament to the dedication of the wound care community to advancing their knowledge and skills,” said Tiffney Oliver, Vice President, Wound Care Learning Network, HMP Education. “For our 2023 meeting, we are excited to offer a world-class lineup of educational sessions as well as a record number of abstracts about the latest research in wound care.”
Submitted abstracts are blind reviewed by a panel of expert judges, based on specific criteria for the category in which it was submitted. Researchers may also be considered for poster grand rounds, oral abstracts, SAWC Young Investigator, and highest scoring abstract honors.
“We are excited to host one symposium for every member of the wound care team, allowing us to provide the highest caliber training and education that all clinicians can incorporate into their practice,” said WHS President Dr. Kenneth Liechty, Division Chief of Pediatric Surgery and Director of Fetal Medicine, University of Arizona, and Surgeon in Chief of Diamond Children’s Hospital. “The quantity and high caliber of the posters presented this year spotlights the most up-to-date research on wound care and limb salvage. This level of exposure to innovation is unparalleled in the wound care community.”
Posters will be on view from 7:30 a.m. to 5 p.m. Friday, April 28, and from 7:30 a.m. to 5 p.m. Saturday, April 29. In addition, SAWC Spring | WHS | DLS participants will have the opportunity to interact with the researchers during the Poster Reception and Awards Presentation from 7:15-8:30 p.m. on April 28, presented by WOUNDS.
Educational Program
The SAWC Spring | WHS | DLS agenda features more than 80 high-impact sessions from expert presenters led by the giants and emerging voices in the field, providing more than 25 CME/CE credits. Participants will have access to sessions in traditional as well as new formats, including hands-on workshops, rapid-fire, case-based, and patient panels. Learning tracks encompass the business of wound care as well as separate tracks through DLS and WHS.
“We have partnered with the Wound Healing Society for 15 years, providing a robust educational experience for meeting participants, and this year will be even stronger with the addition of multiple topics on amputation prevention, said SAWC Spring Co-Chair Dr. Robert S. Kirsner, Harvey Blank Professor and chairman, Dr. Philip Frost Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine. “No other wound care conference offers this level of education, advanced state-of-the-art clinical reviews, and emerging research findings.”
The interdisciplinary agenda is designed for every aspect of wound research, prevention, and healing, with an important focus on limb salvage. Sessions are designed for all members of the wound care team, including physicians, nursing professionals, podiatrists, physician assistants, physical therapists, researchers, scientists, dietitians, and healthcare, sales, and marketing professionals.
For more information or to register, visit sawcspring.com.
ABOUT HMP GLOBAL
HMP Global is the force behind Healthcare Made Practical — and is an omnichannel leader in healthcare content, events, and education, with a mission to improve patient care. The company produces accredited medical education events — in person and online via its proprietary VRTX virtual platform — and clinically relevant, evidence-based content for the global healthcare community across a range of therapeutic areas. Its brands include the HMP Global Learning Network, healthcare’s most comprehensive source for news and information; Psych Congress, the largest independent mental health meeting in the U.S.; the Evolution of Psychotherapy, the world’s largest independent educational event for mental health professionals; the Leipzig Interventional Course (LINC), the leading, global gathering for interdisciplinary cardiovascular specialists; EMS World Expo, North America’s largest EMT and paramedic event; and the Symposium on Advanced Wound Care (SAWC), the largest wound care meeting in the world. For more information, visit hmpglobal.com.
This article was originally published here

Flap Application in Reconstructive Surgery to Manage Severe Radiation-induced Ulcers: A Case Series
Hoang Thanh Tuan Vu Quang Vinh Tran Van Anh Tong Thanh Hai Tran Quang Phu Trinh Tuan Dung


Occam’s Razor Versus Hickam’s Dictum: A Case Report of Junctional Epidermolysis Bullosa
and Lower Urinary Tract Infection Abdur Rehman • Hassan Raza • Beenish Fatima Zia • Fatima Farahi • Meeran Asher Syed

Upcoming Opportunities in Bioengineered Skin Substitutes Market Business Strategies,
Current Insights, Regional Developments, Demand and Forecast to 2030 By Coherent Market Insights Published January 20

Eminently Preventable (amputation)
Jarrod Shapiro, DPM PRESENT Practice Perfect Editor

Examining The Impact of NPWT and Biofilm in Wound Care
Christopher Barrett, DPM, CWS, MAPWCA

The Sandwich Technique for Quick and Efficient Application of Negative Pressure Wound
Therapy to the Feet and Hands: A Case Report Austin Rollins Kristie Ho Luis G. Fernandez Marisse A. Lardizabal Bryan Roth Sean F. O’Keefe Marc R. Matthews

Special Issue on Biomaterials for Wound Repair and Regeneration
Original research articles, review articles and technical communications are welcome. Initial submissions are due by June 1, 2023. Edited By: Boris Hinz, PhD, Robert Kirsner, MD, PhD, Heather Powell, PhD

Wound Care Market Skyrockets, but Still Lacks Standardization
By Rachael Zimlich, RN, BSN
Indigenous Diabetic Foot-Related Lower Extremity Amputations
Integrating Traditional Indigenous and Western Health Models for Improved Outcomes

Wound Healing in Stage IV Pressure Injury With Use of Adjunct Autologous Activated Platelet-rich Plasma Therapy
A Case Report Karina Karina Dinar Rahmania Hubert Andrew Krista Ekaputri Johannes Albert Biben Nungki Ratna Martina

.
January 24th – Register

Spectral MD Announces Positive Interim Results From DFU Clinical Study & Burn Clinical Study Update
DeepView® accuracy increases to 86% in diagnosis of DFU healing potential on day one

Minimally Invasive Surgery as an Evolving Approach to Limb Salvage
Vilayvanh Saysoukha, DPM, DABPM, FACPM, FASPS, AACFAS

‘Who’s In Charge of a Wound?’: Interventionalists Should Play a Role
ISET speakers review the gains to be made by mastering the essentials, with the ultimate goal of avoiding amputation. by Caitlin E. Cox


Biocomposites to host Infection Management Symposium on new best practice framework
for treating diabetic foot infections at Wounds UK – 3 November 2022

Synthetic peptides that form nanostructured micelles have potent antibiotic and antibiofilm activity against polymicrobial infections
Shuli Chou, Huating Guo, Franz G. Zingl, +8, and Xiangyu Mou
Lymphedema Treatment Act – The Frank & Lizzie Show


Arterial Leg Ulcerations Associated With Beta-Thalassemia
Zhisheng Chasen Shao, BA Khurram Khan, DPM

Cochrane Colloquium 2023 to be held in London (UK)
4-6 September 2023 QEII Centre, London, UK

Trauma: The Unrecognized Trigger for Diabetic Foot Ulcers
Mark Hinkes DPM FACFAS FAPWCA DABFAS

Sugar-based inhibitors disarm the pathogen Pseudomonas aeruginosa
Dr. Charlotte Schwenner



Advanced Wound Care Management Market will generate new growth opportunities 2023-2030
Johnson & Johnson Services, Inc., 3M, Baxter

Amputations in Pyoderma Gangrenosum Patients: Underreported or Very Rare?
Shannon Kody, MD Alex G. Ortega-Loayza, MD, MCR

Intranasal Mupirocin to Reduce Surgical Site Infection Post Cardiac Surgery: A Review of the Literature
Jessy Nellipudi, Caleb Stone
Diabetes – A lucrative disease | DW Documentary

Wound Masterclass – Emerging Technologies in Clinical Practice
Negin Shamsian, Chien-Chung Shih, Madelyn R. Larson, Alana M. Mermin-Bunnell, Smiti Mitta, Jian-Cheng Lail, Aref Saberil, Ethan Beard, Serena Jing, Donglai Zhong, Sydney R. Steele, Kefan Sunl, Tanish Jain, Eric Zhaol, Christopher R. Neimeth, Willian G. Viana, Jing Tang, Dharshan Sivaraj, Jagannath Padmanabhan, Melanie Rodrigues, David P. Perrault, Arhana Chattopadhyay, Zeshaan N. Maan, Melissa C.…

Saving Diabetics From Amputations, Chronic Pain, Widely Affordably In …
A Few Weeks Becomes Reality With Circularity’s Groundbreaking Biotech, D’OXYVA Via Upcoming IPO By Rhodri Collins


What’s Trending in Wound Care- Edition 1 Live Webinar February 8
By Nancy Morgan RN, BSN, MBA, WOC

An Explorative Review on Advanced Approaches to Overcome Bacterial Resistance by Curbing Bacterial Biofilm Formation
F Mohamad, Raghad R Alzahrani, Ahlam Alsaadi, Bahauddeen M Alrfaei, Alaa Eldeen B Yassin, Manal M Alkhulaifi, Majed Halwani


Shewanella algae, an Emerging Human Pathogen: A Series of Four Cases From a Portuguese Hospital
Salomão Fernandes, Rita Sérvio, Ana Rita Silva, Raquel Tavares, Paulo Rodrigues

How to Identify Pressure Injuries in Skin of Color
Submitted by Janet Wolfson on January 7th, 2023




REGENATIVE LABS PUBLISHES CASE STUDY DEMONSTRATING NEW APPLICATION OF WHARTON’S
JELLY ALLOGRAFTS IN POST-SURGICAL BREAST REDUCTION CARE


Understanding Racial Disparities in Wound Imaging
Submitted by Alton R. Johnson Jr. on January 6th, 2023




Moisture accumulation detection technologies for identifying pressure injuries: a literature review
Madeline A Bone, Sharon Latimer, Rachel M Walker and Brigid M Gillespie


Expert Insights on Diabetic Peripheral Neuropathy
Jennifer Spector, DPM, FACFAS, Assistant Editorial Director
‘Smart Bandage’ Designed To Help Heal Wounds Faster
By Margherita Cole on December 27, 2022

Keratinocyte Grafts for Dystrophic Epidermolysis Bullosa
Found to Be Safe, Efficacious By Tim Smith

Insight on Platelet-rich Plasma and Applications for Heel Pain
Amanda Westfall, DPM, FACFAS

Stratpharma Webinar – November 4th
A Breakthrough in the Management of Difficult to Heal Diabetic Foot Ulcer – an Innovative Film – Forming Wound Dressing StrataGRT/Stratamed from Stratpharma Switzerland Dr. Shanna Fraser and Dr. Jaminelli Banks

Finite Element Analysis Modelling of Negative Pressure Wound
Therapy Drapes Amy K. McNulty, Robert Wilkes, Jason Bjork, Michael Turnbull, James Sieracki

Best practice, best products, best outcomes in community wound
care: three descriptive cohorts Keryln Carville, Janine Alan and Joanna Smith


Effect of Endovenous Laser Ablation Along With Compression
Therapy on Chronic Venous Ulcer Healing Sheetal Uttaray, Venkata Vineeth Vaddavalli, Ajay Savlania, Arunanshu Behera, Lileswar Kaman, Ujjwal Gorsi

Understanding the Glucagon-like Peptide-1 Receptor
Agonist Medications for Diabetes Robert G. Smith, DPM, MSc, RPh, FNAP

Merry Mistletoe: Healing Value Beyond the Holiday Season
Submitted by Christine Miller

The Effect of a Biofilm-disrupting Wound Gel vs. a Broad-spectrum
Antimicrobial Ointment on a Chronic Wound Microbiome: A Secondary Analysis Associating Clinical and Laboratory Findings Matthew F. Myntti Patricia Stevenson Dianne Porral Valerie Y. Hayes

Journeying Through Progress in Diabetes Treatment:
A Historical Perspective Christine Miller, DPM, PhD

14th Abu Dhabi Wound Care Conference
March 4 & 5, 2023, to be held in ADNEC, Abu Dhabi, United Arab Emirates.

Operating Room Nurses’ Knowledge of and Attitudes About
Pressure Injury Prevention: A Descriptive Cross-Sectional Study Didem Kandemir, PhD Yasemin Ozhanli, PhD, RN Hatice Erdogan, PhD, RN Zeynep Temiz, PhD


A time to unite, heal and innovate
Notes on The Wounds Australia biennial national conference


A Comprehensive Review of the Application of Nanoparticles
in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives Authors Qin W, Wu Y, Liu J, Yuan X, Gao J

Topical vanadate improves tensile strength and alters collagen
organisation of excisional wounds in a mouse model Hendrik Lintel BS, Darren B. Abbas MD, Duncan J. Mackay MD, MBA, Michelle Griffin MBChB, PhD, Christopher V. Lavin MD, Charlotte E. Berry BS, Nicholas J. Guardino BS, Jason L. Guo PhD, Arash Momeni MD, Donald R. Mackay MD, DDS, Michael T. Longaker MD, MBA, Derrick C.…

Dressing ‘revolution’ seeks artificial skin for burn victims
by Marie-Morgane Le Moel with Daniel Lawler in Paris

The effect of high-voltage monophasic pulsed current on diabetic ulcers
and their potential pathophysiologic factors: a systematic review and meta-analysis


The Impact of Youth Onset Type 2 Diabetes on Postoperative
Wound Healing Complications

A Novel Bifunctional Nanoplatform with Aggregation-Induced
Emission Property for Efficient Photodynamic Killing of Bacteria and Wound Healing Authors Hou B, Yang F, Hu C, Liu C, Xiao X, Chen Y, Huang X, Xie S

Evaluation & Management for Office, Outpatient, and Home Visits
Kathleen D. Schaum, MS

Antibiotic-loaded bone substitutes therapy in the management of
the moderate to severe diabetic foot infection: a meta-analysis Xinyi Lin, Yan Wu, Hong Huang, Ruihan Peng, FuHua Huang, Lvrong Hong, WenZhuan Chen

Decreased Health Care Expenditure and Average Length of Therapy
With Facilitated Transition Discharge Program for Patients Receiving Negative Pressure Wound Therapy Leila Boti Laura Soloway Deb Myers Dinu Pillai Javad Zabihi
Fig latex inhibits the growth of pathogenic bacteria
invading human diabetic wounds and accelerates wound closure in diabetic mice

How to assess wound exudate
By Nancy Morgan, RN, BSN, MBA, WOC, WCC, DWC, OMS


Technology in Wound Care
Steven Bowers

Comparison of biopolymer scaffolds for the fabrication of skin substitutes in a porcine wound model
Bronwyn L. Dearman BSc(Hons), PhD, Steven T. Boyce PhD, John E. Greenwood BSc(Hons), MBChB, MD, DHlthSc, FRCS(Eng), FRCS(Plast), FRACS

HOW I TREAT Fungating Wounds
James V. Stillerman, MD, CWSP

Drug-Resistant Aerobic Bacterial Pathogens in Patients with Crocodile Bite Wounds
Attending at Arba Minch General Hospital, Southern Ethiopia Authors Alelign D , Tena T, Tessema M, Kidanewold A
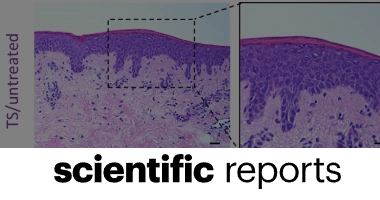
Development of an implantable three-dimensional model of a functional pathogenic multispecies biofilm to study infected wounds
Camila Cárdenas-Calderón, Valentina Veloso-Giménez, Tamara González, Aniela Wozniak, Patricia García, Sebastián San Martín, Juan F. Varas, Ivo Carrasco-Wong, Mario Vera & José Tomás Egaña

Advances in lower limb and foot care study day
Our free advanced study days promote the best practices in skin health and wound healing Sarah Gardner, Jo Dumville, Naseer Ahmed, Leanne Atkin, Rebecca Elwell, Kate Williams
Fibrosis by Karen Ashforth F L Show

Risk Factors and Surgical Outcomes of Diabetic Foot in Diabetic Patients at King Fahad University Hospital
Yasser A. Elghoneimy, Ali A. Alkabah, Hasheem M. Alalsayedsalih, Ali J. Almanyan, Hassan A. Alibrahim, Mostafa H. Albokamsin, Shadi A. Alshammary, Fahd A. Makhdom
Topical Oxygen Therapy Awarded “A” Grade Treatment Recommendation by the American Diabetes Association
in their 2023 Standards of Care in Diabetes
OCEANSIDE, Calif., Dec. 13, 2022 /PRNewswire/ — Advanced Oxygen Therapy Inc. (AOTI), the global leader in noninvasive topical oxygen wound healing solutions, announced today that the American Diabetes Association has awarded an “A” grade recommendation for utilizing adjunctive topical oxygen therapy in treating Diabetic Foot Ulcers (DFU) in their 2023 standards of care in diabetes, the preeminent Clinical Practice Guidance (CPG) in the space, which was published today online: https://diabetesjournals.org/care/issue/46/Supplement_1

ADA – Amputation Prevention Alliance
The American Diabetes Association is the leading clinical authority dedicated solely to combating diabetes and its complications. Based on the latest scientific research and clinical trials, their annually updated standards of care in diabetes provides the most comprehensive and trusted evidence-based clinical guideline on the prevention, diagnosis, and treatment of diabetes and its complications.
Dr. Mike Griffiths, CEO and President of AOTI commented; “We are delighted that the ADA’s Professional Practice Committee, in its 2023 update to their standards of care in diabetes, has assessed that the now overwhelming body of clinical evidence supports awarding topical oxygen therapy a converted “A” grade recommendation as an adjunctive treatment for healing DFU.”
AOTI’s unique Topical Wound Oxygen (TWO2) therapy is unlike any other topical oxygen approach, in that it is the only device that provides a multimodality treatment, combing higher pressure oxygen delivery with non-contact cyclical compression and humidity, in a therapeutic applied by the patient at home. This patented approach has been demonstrated in numerous Randomized Controlled Trial (RCT) and Real World Evidence (RWE) studies to not only heal chronic wounds at a far higher rate, but perhaps more importantly, keep them closed longer term, thereby reducing unnecessary hospitalizations and amputations.1, 2
“The more sustainable long-term healing elicited when utilizing TWO2 therapy was highlighted in the ADA guidance, with their citing of all of the RCT and RWE studies conducted with TWO2, along with multiple recent Systematic Reviews and Meta Analyses, leading to their “A” grade recommendation ” stated Dr. Griffiths
1 Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers; The TWO2 Study. Robert G. Frykberg et al, Diabetics Care 2020; 43:616-624. https://doi.org/10.2337/dc19-0476.
2 Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes; Jessica Izhakoff Yellin, et al; Advances in Wound Care 2022; http://doi.org/10.1089/wound.2021.0118
About AOTI
AOTI is a privately-owned company based in Oceanside, California USA and Galway, Ireland that provides innovative solutions to resolve severe and chronic wounds worldwide. Our products reduce healthcare costs and improve the quality of life for patients with these debilitating conditions. Our patented non-invasive Topical Wound Oxygen (TWO2) homecare therapy is clinically proven to deliver Sustained Wound Healing that reduces both Amputations and Hospitalizations, So Life Can Get Back to Normal.
For more information see: www.aotinc.net
Contact:
Dr. Mike Griffiths
CEO & President
350571@email4pr.com
(760) 672 1920
This article was originally published here
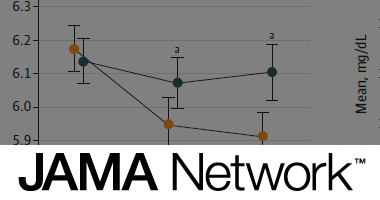
Effects of a Low-Carbohydrate Dietary Intervention on Hemoglobin A1c
Kirsten S. Dorans, Lydia A. Bazzano, Lu Qi

Caring for Atypical Wounds in Patients With Severe Obesity A Case Series
Matthew Ciabattoni, DNP, FNP-BC, CWOCN Amanda C. Ward, BSN, RN-BC, CWON Ave Maria Preston, MSN, RN, CWOCN, ACNS-BC

Skin Substitute Reimbursement and Coding Best Practices Webinar Series
Dr. Jeffrey Lehrman and Terrence Mabry

The role of non-medicated dressings for the management of wound infection
Author(s) : Thomas Bjarnsholt, Val Edwards-Jones, Matthew Malone, Karen Ousey, Mark Rippon, Alan Rogers, Samantha Westgate, Sabine Eming, Isabelle Fromantin, Astrid Probst, Hans Smola, Hui-Mei Yang, Jiun-Ting Yeh, Steven Percival

Effectiveness of Negative Pressure Wound Therapy in Patients With Challenging Wounds: A Systematic Review and Meta-analysis
Mohamed S.A. Shehata Eshak Bahbah Yousef El-Ayman Ahmed H. Abdelkarim Ahmed R. Abdalla Ahmed Morshedy Khaled Turkmani Ishith Seth Nimish Seth


Benefits of a Portable, Mechanical Negative Pressure Device for Pediatric Patients
Elizabeth Day Dechant, BSN, RN, CWOCN, CFCN


Active treatment of non-healing wounds in the community
Author(s): Jacqui Fletcher, Jaqueline Dark, Luxmi Dhoonmoon, Caroline Dowsett, Jeanette Milne, Holly Robinson, Andrew Sharpe

Transforming Cleansing Practice: Following the Science and Consensus Guidelines – Webinar
Joyce Black, PhD RN FAAN Elizabeth Faust, MSN, CRNP, CSWS, CWOCN-AP, MAPWCA

Macronutrient and micronutrient intake of individuals with diabetic foot ulceration
Rebecca Collins, Tracy Burrows, Hailey Donnelly, Peta Ellen Tehan
Yes, It Is Possible to Apply for New ICD-10-CM Codes!
Schaum, Kathleen D. MS

Use of a New Dressing Protocol in Chronic Lower Extremity Wounds on Homebound Patients: A Retrospective Study
Arturo Gonzalez, DNP, APRN, ANP-BC, CWCN-AP

Reflecting on the Past, Present, and Future of Diabetic Foot
Jennifer Spector, DPM, FACFAS, Assistant Editorial Director

Comparison Between Numeric Parameters From Acute and Chronic Venous Leg Ulcers
Identification of Potential Objective Nonhealing Parameters Nicolas A. Cerusico, PhD1; Romina Chavez-Jara, PhD1; Silvana A. Lopez, MD2; Eugenia Sesto Cabral, PhD1; Aida Ben Altabef, PhD3; and Alberto N. Ramos, PhD1

Managing Exudate Pooling: A Simplified Practical Guide
Emily Greenstein, APRN, CNP, CWON-AP, FACCWS1; Maarten Vooijs, MSc2; and Sarah Norton, MSN, MBA, RN, CWCN3

Recent Advances in Nanozymes for Bacteria-Infected Wound Therapy
Fayin Mo, Minjun Zhang, Xuewei Duan, Chuyan Lin, Duanping Sun, Tianhui You

Acetic Acid and Wound Healing
Ilya Petrou, MD



Knowledge, Awareness, and Practice Related to Diabetic Foot Ulcer Among Healthcare Workers
and Diabetic Patients and Their Relatives in Saudi Arabia: A Cross-Sectional Study Sultan H. Alsaigh, Raneem H. Alzaghran, Dalal A. Alahmari, Lama N. Hameed, Kadi M. Alfurayh, Khozama B. Alaql
MolecuLight Featured in Vizient Tech Watch as Key Technology for Visualizing Bacterial Burden
and Helping to Reduce Surgical Site Infections
Article is a Follow-On to MolecuLight’s Receipt an Innovative Technology Contract from Vizient Last Year
PITTSBURGH, Dec. 6, 2022 /PRNewswire/ – MolecuLight Corp., the leader in point-of-care fluorescence imaging for detection of wounds containing elevated bacterial loads, is featured in Vizient’s newly released Tech Watch publication as a key technology for visualizing bacterial load and its locations, and helping to reduce surgical site infections. The article, “Fluorescence Imaging: New technology enables point-of-care surgical wound bacterial assessment” is featured in Vizient’s Tech Watch (Medical Device) Volume 3 issue, issued this past week.
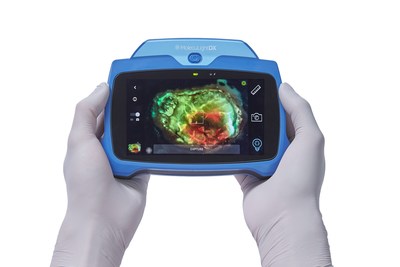
MolecuLightDX™ point-of-care Imaging device to detect elevated bacterial loads in wounds to help clinicians prevent surgical site complications. (CNW Group/MolecuLight)
The Tech Watch article describes how surgical site infections (SSIs) occur in up to 38% of surgeries1 (depending on anatomical location and type of surgery) and account for 20% of all healthcare-acquired infections2. SSIs are also the costliest of these infections, extending the average length of hospital stays by 9.7 days and costing more than $20,000 per patient admission3,4.
Early and accurate diagnosis of post-surgical bacterial loads and infection is critical to enable prompt treatment before the infection worsens. Some cases require lab testing to accurately diagnose the bacteria colonizing the wound, allowing the offending bacteria to grow and spread and delay effective treatments. Test results can take days to weeks to be available and, if positive, could be too late to prevent infection.
Clinicians need real-time diagnostic tools that they can use at the point-of-care to help provide immediate information on the state of the wound and possible growth of bacterial burden. The article argues that MolecuLight imaging helps eliminate unnecessary subjectivity in assessing wounds for the presence of harmful bacteria by allowing quick and accurate visualization of locations of elevated bacteria load in wounds, along with clinical signs and symptoms. As such, it provides “invaluable real-time information to inform clinical decision-making”.
A recent per-reviewed study5 supports this position in demonstrating the benefits of using MolecuLight to help clinician visualize bacterial burden in surgical site wounds:
- 76% of surgical sites in the study that reach the stage of referral to a wound specialist had clinically significant bacterial loads (104 to 109 CFU/g), however only 6.8% exhibited symptoms of infection, resulting in delayed infection management.
- Point-of-care fluorescence imaging (using the MolecuLight i:X device) for detecting high bacterial loads improved sensitivity by 5.7-fold compared to clinical signs and symptoms alone.
- Clinician experience with fluorescence imaging and interpretation (>200 imaging sessions) increased sensitivity of fluorescence imaging to 11.3-fold higher than clinical signs and symptoms alone, and accuracy to 2.6-fold higher.
“Clinicians need an objective means of detecting infection or another surgical wound complication without having to rely on subjective judgment,” says Kylie Sandy-Hodgetts, PhD, Founder and inaugural President of the International Surgical Wound Complications Advisory Panel (ISWCAP).
“Fluorescence imaging using MolecuLight is positioned to change contemporary paradigms of post-surgical wound management due to its ability to quickly and reliably detect bacterial burden and visualize contamination at the point-of-care”.
In addition to the profile in Vizent’s Tech Watch, last year the MolecuLight i:X® fluorescence wound imaging device received an Innovative Technology contract from Vizient, Inc., the nations’ largest member-driven health care performance improvement company. The new Innovative Technology contract for MolecuLight i:X signifies to Vizient members the device’s unique qualities that potentially bring improvement to the health care industry.
“We have been working with MolecuLight since 2021 when Vizient recognized the company as an awarded supplier through our Innovative Technology Program,” said Tami Maurer, VP, Contract & Program Services at Vizient, Inc. “Carefully evaluated and selected by our member council of clinical and supply chain professionals, Vizient’s Innovative Technology Program recognizes innovative advancements in care, enabling healthcare providers to offer the highest quality care while encouraging manufacturers to continue to pioneer new solutions.”
The MolecuLight i:X and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 60 peer-reviewed publications involving 1,500 patients, they are used by leading wound care facilities globally.
About MolecuLight Corp.
MolecuLight Corp. is the US subsidiary of MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight ‘s suite of commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
This article was originally published here

A Practitioner’s View on the Concept of Aging
Tracey Rickards, BN, RN, MN, PhD Chris Boodo, MIMOSA Diagnostics

Treating Smaller Wounds with Disposable NPWT
Robert Klein, DPM, FACFAS, CWS, FFPM RCPS (Glasgow)

Microchannel-containing nanofiber aerogels with small protein molecule enable accelerated diabetic wound healing
Terasaki Institute for Biomedical Innovation



How a Fish Skin Helped Heal Soft Tissue Deficits in Infected Wounds
Trenton Leo, DPM; Richard Bruno, DPM, AACFAS

Working Smarter, Not Harder: Strategies for Wound Management Success
Wednesday, December 14, 2022 | 1:00 – 2:00pm ET

Effects of vildagliptin on wound healing and markers of inflammation in patients with type 2
diabetic foot ulcer: a prospective, randomized, double-blind, placebo-controlled, single-center study

Building wound care capability: the development of an education and training directory for health professionals in Australia
Christina Parker, Andrew Francis, Laura Robson and Angela Jones

The use of gentian violet and methylene blue impregnated foam for treatment of chronic leg ulcers
Alexander Savage, Lisa J Ellis and Patricia Terrill



Buruli Ulcer and Medical Geo-Microbiology
Aseer Manilal, Dagimawie Tadesse, Kuzhunellil Raghavanpillai Sabu


A not so new treatment in wound care
By Mia Smitt Tucson Local Media Columnist




Latest Pearls in Wound Healing
November 20, 2022 Carrie Nagorka, Senior Editor

Fungating Malignancies: Management of a Distinct Wound Entity
Kondra, Katelyn MD; Pekcan, Asli BS; Stanton, Eloise BA; Cook, Austin D. BS; Jimenez, Christian BS; Aronowitz, Alexandra BS; Winterhalter, Bridget A. PA-C, MPH; Hammoudeh, Jeffrey A. MD, DDS; Aronowitz, Joel A. MD

Nutrition Interventions in Adults with Diabetic Foot Ulcers
Last updated November 3, 2022


Robert Kirsner MD, PhD: Presentation Highlights from ‘Healing the Unhealable Wound’
An interview at the SDPA 2022 Conference on major talking points from Dr. Robert Kirsner’s presentation on wound care with dermatologists and physician associates.

Qualitative exploration of patient and healthcare professional perspectives on
barriers and facilitators to foot self-care behaviors in diabetes Andrew Hill1, Mairghread Ellis, Fiona Gillison

Risk factors for impaired wound healing and wound complications
Authors:David G Armstrong, DPM, MD, PhD Andrew J Meyr, DPM Section Editors: Russell S Berman, MD John F Eidt, MD Joseph L Mills, Sr, MD Amalia Cochran, MD, FACS, FCCM Deputy Editor:Kathryn A Collins, MD, PhD, FACS




Franklin W. Harry, DPM, is recognized by Continental Who’s Who
ST. LOUIS, Nov. 15, 2022 /PRNewswire/ — Franklin W. Harry, DPM, is being recognized by Continental Who’s Who as a Trusted Podiatry Surgeon for his exemplary work in the Medical field, acknowledging his private practice achievements at Best Foot Forward.

Franklin Harry, DPM, ABMSP, is the founder of Best Foot Forward
A skilled, board-certified podiatrist, Dr. Harry is the founder of Best Foot Forward, with offices located in Festus and St. Louis, MO. With 13 years of experience practicing medicine, he enjoys working in all areas of foot and ankle care, specializing in diabetic wound care and foot and ankle surgery. His other areas of expertise include bone deformities and arthritis, and foot and ankle injuries.
Podiatry is a branch of medicine devoted to the study of diagnosis and medical and surgical treatment of various disorders of the foot, ankle, and lower extremities. A podiatrist, also known as a podiatric physician or a foot and ankle surgeon, is a medical professional devoted to treating disorders of the foot, ankle, and lower extremities. They can treat injuries and complications from ongoing health issues like diabetes.
Best Foot Forward (BFF) is committed to providing excellent podiatric care by enhancing the quality of life of patients with foot, ankle, and leg problems. BFF strives to preserve and restore the health of the lower extremities and provide patient-centered care. Their podiatrists treat everything from difficult to trim toenails, calluses, warts, foot infections, diabetic wound care, fall prevention, and surgical foot and ankle repairs.
Before starting his medical career, Dr. Harry earned his Doctor of Podiatric Medicine degree from Barry University in Miami, FL. He completed his surgical residency at Wyckoff Heights Medical Center and received advanced training with the Central Kentucky Diabetes Fellowship, specializing in complex wounds, limb salvage, and the biomechanics of fall prevention.
An authority in his field, the doctor is a member of the American Association of Podiatric Practice Management and is board-certified by the American Board of Multiple Specialties in Podiatry. He is a member of the American Board of Podiatry, the Relias Wound Care Institute, the Missouri Podiatric Medical Association, and the Illinois Podiatric Medical Association.
Outside of his practice, the doctor volunteers with Peter and Paul and Biddel House homeless shelters, providing podiatric medical care for the less fortunate. In addition, the doctor goes on mission trips to Haiti with Hands Helping Haiti and to Guatemala with Washington University.
In his free time, Dr. Harry enjoys spending time with his wife, Misty Gonzalez, Pharm. D., to whom he has been married since 2017. They have one child and a dog named Bentley. They enjoy cooking, hiking, scuba diving, exploring new restaurants, and are avid sports fans.
In light of this recognition, Dr. Harry wishes to thank his mentors: Ronald Guberman, DPM, Jonathan Moore, DPM, and Pamela Jensen, DPM.
For more information, visit www.bffdocs.com.
This article was originally published here

Use of Citrulline as an Efficient Arginine Supplement
Nancy Collins, PhD, RDN, LD, NWCC, FAND