Category: Articles


What Happens When a Wound Heals?
Featuring Alton Johnson, DPM and John Steinberg, DPM

The Frank & Lizzie Show Episode 017: Oxygen CARES: Solid Evidence that Oxygen Helps Heal Wounds
89 views Jul 12, 2023 Frank & Lizzie were LIVE in National Harbor, MD speaking with Dr Matthew Garoufalis, DPM, FASPS, FACFAOM, CWS on his experience with the use of Topical Oxygen Therapy (TOT) using Advanced Oxygen Therapy, Inc. (AOTI). Dr Garoufalis will highlight the latest evidence to support the use of TOT in our…


AOTI Participates in Panel Discussion at Preventing Diabetes-Related Amputations in America
A Solutions Summit Organized by the American Diabetes Association’s Amputation Prevention Alliance

The Prevalence of Anemia in Hospitalized Patients With Diabetic Foot Ulcer (DFU)
and the Relationship Between the Severity of Anemia and the Severity of DFU Ritesh Kumar • Surya K. Singh • Neeraj K. Agrawal • Ujwal Kumar • Subhash Kumar • Supreeth C • Avina Bishnoi

An Immunomodulatory Biomimetic Single-Atomic Nanozyme for Biofilm Wound Healing Management
Juyang Zhang, Mengdi Lv, Xinye Wang, Fan Wu, Cheng Yao, Jian Shen, Ninglin Zhou, Baohong Sun

2023 Annual Scientific Symposium Wound Healing: October 12-15, 2023
Knowledge, Diagnosis & Management Philadelphia, PA

Diabetic Foot Ulcers – A Review
David G. Armstrong, DPM, MD, PhD; Tze-Woei Tan, MBBS, MPH; Andrew J. M. Boulton, MD, DSc

Transverse Tibial Bone Transfer in the Treatment of Diabetes Foot Ulcer: A Pilot Study
Rongzhi Wen,* Xinhua Cheng,* Hong Cao, Lei Zhang, Fangcheng Luo, Wei Shang



Negative Pressure Wound Therapy Promotes Wound Healing by Inhibiting Inflammation in
Diabetic Foot Wounds: A Role for NOD1 Receptor

Using WHO tools to influence surgical infection prevention
Wounds Week 6 – Using WHO tools to influence surgical infection prevention // Making an impact on surgical site infection in the post-operative phase


A Case Report of Localized Vegetative Pyoderma Gangrenosum of the Foot in a
Patient With Myasthenia Gravis in an Outpatient Setting Zeib Syed • Shahrukh Syed • Katherine Burmaster

Synergistic Effects of Pure Hypochlorous Acid and Ovine Forestomach in Chronic Venous Disease
M. Mark Melin Monika Gloviczki Peter Gloviczki Abigail Chaffin Lee Ruotsi

Reconstruction of Foot and Ankle Defects: A Prospective Analysis of Functional and Aesthetic Outcomes
Michael Laitonjam • Manal M. Khan • Deepak Krishna • Ved Prakash Rao Cheruvu • Reena Minz

Diabetes Dialogue: Reforming Language in Metabolic Disease, with Jane Dickinson, RN, PhD
Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES Natalie Bellini, DNP, FNP-BC, BC-ADM, CDCE

How Does a Wound Dressing With Sensors Monitor the Healing Process?
Armin Haas Prof Kai-Uwe Zirk



Diabetes Dialogue: Diabetic Foot Ulcers and Social Determinants of Health
Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES Natalie Bellini, DNP, FNP-BC, BC-ADM, CDCE
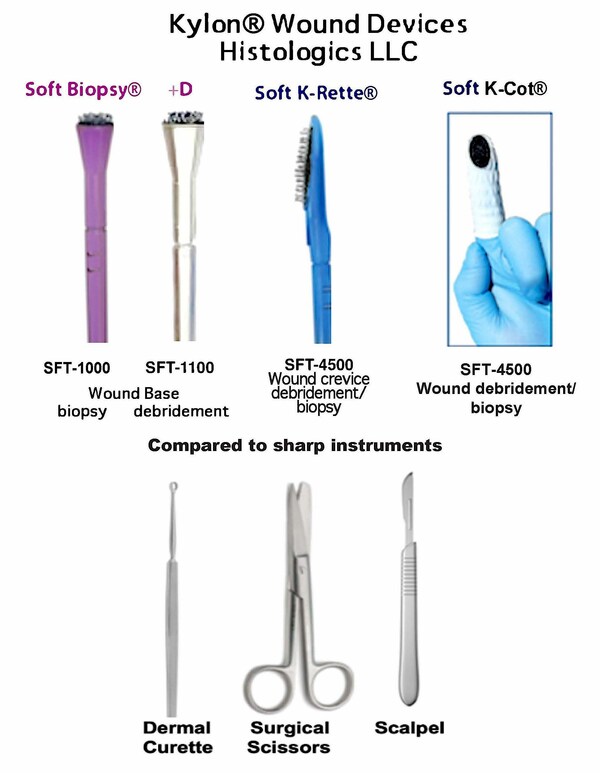
Histologics LLC Releases SoftBiopsy®+D, the Versatile Kylon® Fabric-Tipped Device for Wound Debridement or Tissue Biopsy Sampling
ANAHEIM, Calif., July 5, 2023 /PRNewswire/ — Histologics LLC attended “WOCNext” in Las Vegas last June 4-6th to exhibit its novel debridement and wound biopsy fabric-curettage devices including for the first time, the SoftBiopsy®+D device. Over 1M cases by clinicians used Kylon® devices for colposcopic biopsy and curettage in the USA, in nearly every clinical setting www.histologics.com. The hooked medical (Kylon®) fabric tipped brushes gently remove and can trap tissue for biopsy for histology or microbiology. The debridement may be performed at a lighter “hygiene” level, extending all the way to “excisional” surgical methods when the brush array converts to micro-curettes if the fabric is pressed firmly and wiped or twisted into tissue.
Kylon® Fabric Debridement and Biopsy : Video Simulation
The cause of some wounds not to heal includes biofilm, and the Kylon® fabric devices can be used to obtain true tangential biopsy samples from the debrided wound base for lab testing. This is commonly done with the SoftBiopsy® product, solely used for biopsy that is sent to a laboratory. Histologics recently released SoftBiopsy®+D, a more versatile and durable version for clinicians, that can sustain longer procedure time for wound debridement as well as biopsy.
We have previously demonstrated the value of our Soft K-Rette® device to debride and sample crevice wounds, and Soft K-Cot® deployed on the finger for “Compassionate Debridement at Your Fingertips®”.
The scope of practice of most physicians and nurse clinicians vary from basic wound care to the most advanced surgical procedures where necrotic wound tissue must be mechanically or surgically removed. Advanced and basic wound care providers include many specialties in health care and procedures occur in clinics, hospitals, homes, facilities and other settings. Some providers that shy away from regular debridement (necessary to heal wounds) due to the invasive nature of the scalpel and sharp curette would be willing to clean wounds using the gentle Kylon® devices.
Please visit our wound care website to request free samples at www.histologicwc.com, or contact Lily Ramos at histologicswc@gmail.com, Toll Free: 888-235-2275.
SOURCE Histologics LLC

Factors Affecting Platelet-Rich Plasma Success in Patients With Diabetic Foot Ulcer
Eyüp Murat Kanber • Harun Gulmez

Metabolic memory in diabetic foot syndrome (DFS): MICRO-RNAS, single nucleotide polymorphisms
(SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction Alessandro Del Cuore, Rosaria Maria Pipitone, Alessandra Casuccio, Marco Maria Mazzola, Maria Grazia Puleo, Gaetano Pacinella, Renata Riolo, Carlo Maida, Tiziana Di Chiara, Domenico Di Raimondo, Rossella Zito, Giulia Lupo, Luisa Agnello, Gabriele Di Maria, Marcello Ciaccio, Stefania Grimaudo & Antonino Tuttolomondo

The use of sodium-glucose co-transporter-2 inhibitors or glucagon-like peptide-1 receptor
agonists versus sulfonylureas and the risk of lower limb amputations: a nation-wide cohort study Nikki C. C. Werkman, Johanna H. M. Driessen, Coen D. A. Stehouwer, Peter Vestergaard, Nicolaas C. Schaper, Joop P. van den Bergh & Johannes T. H. Nielen
Wound Masterclass Podcast | Episode Five: Wound Care in the Military Setting
The Wound Masterclass Podcast brings you our fifth episode! This time the topic is ‘Wound Care in the Military Setting’ and we are joined by two guests to share their extensive experience in the area : Lt Col Professor Steven Jeffery and Dr Aliza Lee.

Elimination of methicillin-resistant Staphylococcus aureus biofilms on titanium
implants via photothermally-triggered nitric oxide and immunotherapy for enhanced osseointegration

A Retrospective Study on the Clinical, Laboratory, and Nutritional Status of Pediatric Epidermolysis Bullosa in a Tertiary
Referral Hospital in West Java, Indonesia

A Rare Case of Locally Contracted Cutaneous Corynebacterium Diphtheriae
Marc Trubin • Sarah E. Eichinger • Annette Abraham • Shivanjali Shankaran

ALLOGRAFT LITIGATION MOVED UP TO FEDERAL COURT
KIM DELMONICO

Innovative paper-like, battery-free, AI-enabled sensor for holistic wound monitoring
Low-cost and versatile patch can be customised for different types and sizes of wounds, and provides early warning of adverse conditions to facilitate wound care and management

Revolutionizing Podiatry for Efficient Patient Care
As the director of podiatric medical education and co-chief of the podiatry division, I lead certified residency and fellowship training in wound care along with our parent organization, Council on Podiatric Medical Education. We are a renowned healthcare organization specializing in podiatry and a broad range of primary care and specialty services …

The Mad Hatter’s Tea Party, Pressure Injury Reporting & Perverse Incentives
by Caroline Fife, M.D. | Jun 22, 2023


Bebe Rexha’s Wound Care; ‘The System Is Broken’; Finding Nemo in a Blood Drain
Healthcare social media content from around the Web gathered by MedPage Today staff
Nuo Therapeutics’ Aurix® System Added To Wound Care Formulary Of Wound Care Advantage
HOUSTON, June 22, 2023 (GLOBE NEWSWIRE) — Nuo Therapeutics, Inc. (OTCQB: AURX) (“Nuo”), a commercial stage medical device company pioneering leading-edge biodynamic therapies by focusing on emerging opportunities in the evolving healthcare landscape, is pleased to announce that Wound Care Advantage (WCA), the nation’s leading wound care consulting firm has added the Aurix® System to its formulary. Founded in 2002, Wound Care Advantage (WCA) has established a large network of successful wound healing programs with partner hospitals. Through a strong commitment to quality care and innovation, WCA has built financially sustainable wound care programs that have saved limbs and lives of more than 40,000 patients suffering chronic wounds.
“Diabetic foot ulcers pose a significant risk to patients and can be challenging for wound care centers to treat from both clinical and financial perspectives,” commented Dave Hazard, Nuo’s Vice President of Sales. “With thousands of commercially available wound care products, it can be extremely difficult for wound care centers to identify products that are both reimbursed by Medicare, and more importantly, that actually heal patients. We are excited to partner with Wound Care Advantage’s team of experts who rigorously vet each product that is placed on the formulary.”
The Platelet Rich Plasma gel produced by the Aurix System is cleared by the FDA for treating chronic wounds with a simple one-minute spin. In a clinical study performed with the Centers for Medicare and Medicaid Services (CMS), the Aurix System demonstrated a higher healing rate and a significant time to heal advantage as compared to other advanced healing modalities.
About Nuo Therapeutics
Nuo Therapeutics, Inc. is a commercial stage medical device company pioneering leading-edge biodynamic therapies by focusing on emerging opportunities in the evolving healthcare landscape. The Company’s Aurix System is a biodynamic hematogel that harnesses a patient’s innate regenerative abilities for the management of a variety of wounds.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements. These forward-looking statements may include statements that are predictive in nature and depend upon or refer to future events or conditions, and may include words such as “believes,” “plans,” “anticipates,” “projects,” “estimates,” “expects,” “intends,” “strategy,” “future,” “opportunity,” “may,” “will,” “should,” “could,” “potential,” or similar expressions. You are cautioned not to unduly rely on forward-looking statements. Forward-looking statements are based on current expectations, assumptions, and information available to the Company’s management and are subject to known and unknown risks, uncertainties and other factors which may cause actual results to differ materially from the forward- looking statements. These risks, uncertainties, and factors are discussed under “Risk Factors” and elsewhere in the Company’s public filings with the U.S. Securities and Exchange Commission from time to time, including the Company’s annual report on Form 10-K, quarterly reports on Form 10-Q, and current reports on Form 8-K. You are advised to carefully consider these various risks, uncertainties, and other factors. The Company expressly disclaims any intent or obligation to update or revise publicly these forward-looking statements except as required by law.
Contact:
David Jorden
djorden@nuot.com

Divide and Conquer: Resistant Bacteria Splits up to Infect Hospital Patients
Pseudomonas aeruginosa forms two subpopulations to improve colonization success Division is controlled by a genetic switch that affects c-di-GMP levels Identification of this division mechanism can aid in treatment strategies
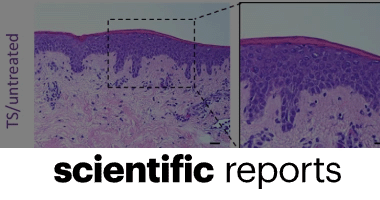
HOXA3 accelerates wound healing in diabetic and aged non-diabetic mammals
K. Parella, K. Moody, D. Wortel, H. Colegrove & J. A. Elser
Under Pressure: Shedding Light on the Issue of Pediatric Pressure Injury Treatment
Bay, Caroline C. BA; Grush, Andrew E. BS; Buchanan, Edward P. MD, FACS; Highfield, Linda PhD, MS; Krasnosky, Ryan MPAS, PA-C, DFAAPA, DrPH

Vascular Surgery In Diabetes Management: Not Just It Prevents Limb Loss But Improves Overall Quality Of Life
Individuals with diabetes are at an increased risk of peripheral arterial disease (PAD), which can result in critical limb ischemia. Vascular surgery can prevent unnecessary limb amputations in individuals with critical limb ischemia. Written by Longjam Dineshwori
Episode Five: Wound Care in the Military Setting
he Wound Masterclass Podcast brings you our fifth episode! This time the topic is ‘Wound Care in the Military Setting’ and we are joined by two guests to share their extensive experience in the area : Lt Col Professor Steven Jeffery and Dr Aliza Lee.

Recent Strategies and Future Recommendations for the Fabrication of Antimicrobial, Antibiofilm, and Antibiofouling Biomaterials
Shakeel Ahmad Khan, Adnan Shakoor

Transcriptomics-driven drug repositioning for the treatment of diabetic foot ulcer
Wirawan Adikusuma, Zainul Amiruddin Zakaria, Lalu Muhammad Irham, Baiq Leny Nopitasari, Anna Pradiningsih, Firdayani Firdayani, Abdi Wira Septama & Rockie Chong


Tuberculous Osteomyelitis of the Left Fifth Metatarsal and Phalanx
Without Pulmonary Involvement: A First-of-Its-Type Report Sankalp Yadav • Gautam Rawal • Madhan Jeyaraman

Helping The Patient Throughout A Wound Care Journey (audio)
Morgan McCoy and Caroline Fife, MD, discuss Morgan’s wound care experience after a lower extremity amputation, and what physicians need to know about providing the best experience for their patients.
Seasoned Healthcare Executive David Bassin Joins the MolecuLight Board of Directors
Former Healogics CEO Brings Deep Wound Care Industry Experience to MolecuLight’s
Rapidly Growing Business
TORONTO, July 6, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated, pathogenic bacterial loads in and around wounds, is pleased to announce the appointment of David Bassin to its Board of Directors as an Independent Board Member.
David Bassin brings a wealth of expertise and experience in the healthcare industry, particularly in the field of wound care. As the founder of GIO Advisory LLC, he has provided invaluable advisory services to numerous companies and private equity firms based on his extensive background in healthcare, spanning pharmaceutical/device, payer, and provider services. Most recently, Bassin served as the CEO of Healogics, the foremost provider of wound care services in the US, operating over 630 wound care centers and 300 providers. During his tenure, he successfully restructured and refocused the business, resulting in strong earnings growth prior to his transition into advisory services. Prior to his role as CEO, Bassin served as the CFO of Healogics, contributing to the company’s financial growth and success. Bassin’s impressive career also includes significant roles in other healthcare organizations. He served as the CFO of eviCore Healthcare, Inc., where he oversaw multiple dividend recapitalizations and facilitated a merger with the company’s largest competitor, leading to the creation of a company with over 3,000 employees. Additionally, Bassin held the position of CFO at InVentiv Health, Inc., a provider of services to pharmaceutical companies, where he successfully managed a high-growth company and orchestrated a go-private transaction valued at over $1 billion and delivered a significant premium to its shareholders.
“We are thrilled to welcome David Bassin to the MolecuLight Board of Directors,” said Anil Amlani, CEO of MolecuLight. “His extensive experience in optimizing and scaling organizations across the healthcare industry, particularly in wound care, will be invaluable to MolecuLight as we continue to expand our global presence. Our MolecuLight devices have already become indispensable tools in wound assessment and real-time decision-making for thousands of clinicians worldwide. David’s expertise will greatly support our mission to meet the increasing global demand for our innovative wound care diagnostics and establish them as the gold standard in the field.”
David Bassin expressed his excitement about joining MolecuLight’s Board of Directors, stating, “There are over 6.5 million patients living with wounds. As an industry, we need to continue to develop new solutions that improve wound care treatment effectiveness and efficiency. With a global drive to improve outcomes, reduce costs, and minimize antibiotic usage, MolecuLight’s point-of-care devices have demonstrated their ability to effectively address these critical clinical needs. I am deeply impressed by the organization, the technology’s alignment with market demands, and the significant market traction they have achieved. I am eager to contribute to their growth and help them achieve their ambitious goals.”
MolecuLight’s groundbreaking i:X® and DX™ imaging devices are the only FDA-cleared and CE and Health Canada approved devices for the real-time detection of elevated bacterial burden in wounds. Supported by over 80 peer-reviewed publications involving 2,600 patients, these devices are widely utilized by leading wound care facilities worldwide.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
For more information, contact:
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
T. +1.647.362.4684
rsandler@moleculight.com
www.moleculight.com
Image:
SOURCE MolecuLight


Peptides For Healing | Ultimate Guide In 2023
Several peptides have stood out from the rest for their potential benefits in wound healing, tissue repair, and regeneration. These best healing peptides include BPC-157, Thymosin Beta-4/TB500, Melanotan 2 (II), Sermorelin, and GHK-Cu

Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment
by Satish Sharma,James Mohler, Supriya D. Mahajan,Stanley A. Schwartz, Liana Bruggemann, Ravikumar Aalinkeel


Scientists discover small RNA that regulates bacterial infection
GEORGIA INSTITUTE OF TECHNOLOGY


Chronic wound healing using glass
UNIVERSITY OF BIRMINGHAM
The Wound Company Launches With $4.25M In Funding To Curb the Amputation Epidemic And Save The Healthcare System Billions
Led by Susa Ventures and Sozo Ventures, the funding will be used to transform the $45B wound and ostomy care industry by bringing on-demand experts to more patients and providers in need via telehealth and in-person visits
The Wound Company, a multi-channel on-demand wound and ostomy care delivery company that improves patient outcomes, today announced its launch from stealth with $4.25M in seed funding from Susa Ventures and Sozo Ventures. The funding will be used to expand the company’s national footprint, hire top talent, and to continue improving health outcomes in the most cost-effective way possible while bringing dignity to the over 13 million people in need of improved wound and ostomy care.
Why Wound Care Matters
The US is experiencing an amputation epidemic due to diabetic foot ulcers and other serious wounds. Despite medical advancements, Americans are amputating double the number of limbs today than during the Civil War. About 50% of lower extremity amputations would have been preventable if patients with type 2 diabetes and foot ulcers had access to better healthcare. This issue is one of the problems The Wound Company is solving for.
Often providers need more wound care expertise or, due to understaffing, don’t have time to offer comprehensive care continuously, leaving patients to figure it out on their own. This leads to wound care patients returning to the hospital due to improper wound care. But it can be prevented.
“The wound and ostomy care industry is broken,” said Nima Ahmadi, founder and CEO of The Wound Company. “It’s operating in the fee-for-service world, which pushes expensive procedures and products that help the bottom line, but don’t impact outcomes for the patient. We’re paid to heal wounds with continuous care and, in doing so, save money for health plans and at-risk providers.”
Enter The Wound Company
The Wound Company aims to fix the broken space of wound and ostomy care by using predictive analytics and multi-channel communications to deliver the right wound and ostomy care to the right patient in the right channel at the right time. The tech connects patients and providers to wound and ostomy experts virtually or via in-person visits to ensure they have top-tier care.
“The Wound Company’s innovative technologies have the potential to save health plans billions of dollars and transform the patient experience,” says Susa Ventures Investor Derick En’Wezoh. “With a dedicated team of highly experienced experts, a strong vision, and a passion for improving healthcare outcomes, this tech will save lives.”
The platform also offers clinical reporting, customer data integration, and workflow automation to make care delivery as painless as possible for providers.
While in stealth, The Wound Company has already partnered with health plans, health systems, home care providers, hospice providers, and patients, with significant results to date:
- A potential 15-20% reduction in the total cost of care for wound and ostomy patients for payers
- Up to 50% savings on supplies per patient for home and hospice care providers
- 60% of patients demonstrated progressive healing week after week
- 90% of Stage I/II pressure ulcers resolved without advancing to a higher stage
- 100% of ostomy patients have a predictable pouching system and reduced chance of ER visits or readmissions
“Our blend of virtual and in-person services provided by passionate experts in wound care helps people heal quickly, safely, and with the dignity they deserve while helping to alleviate the pressure on overworked healthcare professionals,” said Chief Medical Officer Sanford Roberts.
The Wound Company is open to partnerships with health plans, at-risk providers, home health providers, and hospice care providers. For more information, visit www.thewound.co.
About The Wound Company
The Wound Company is a Minneapolis-based technology company dedicated to advancing wound and ostomy care. The company uses proprietary technology to connect providers with experienced and certified wound care specialists who can care for patients virtually or via in-person visits. The Wound Company partners with health plans, home care companies, and providers to bring dignity to patients with wounds and ostomies while increasing positive patient outcomes.
This article was originally published here

Evaluation & Management for Office, Outpatient, and Home Visits
Kathleen D. Schaum, MS

The impact of youth-onset type 2 diabetes on postoperative wound healing complications
Hilliard T. Brydges BS, Grace McDonnell, Hani Y. Nasr MD, Bachar F. Chaya MD, Ogechukwu C. Onuh BA, Allyson R. Alfonso MD, Daniel J. Ceradini MD

Recent Advances in Nanozymes for Bacteria-Infected Wound Therapy
Fayin Mo, Minjun Zhang, Xuewei Duan, Chuyan Lin, Duanping Sun, Tianhui You

The effect of high-voltage monophasic pulsed current on diabetic ulcers and their potential
pathophysiologic factors: A systematic review and meta-analysis Beshoy Girgis PT, MSc, Davide Carvalho MD, PhD, José Alberto Duarte MD, PhD

Chitosan Sponges Are Associated With Higher Rates of Wound Complications Compared to Calcium Sulfate Beads
Kelsey McKee • Joseph Easton • Brian Mullis • Ivan Hadad

Antibiotic-loaded bone substitutes therapy in the management of the moderate to severe diabetic foot infection: A meta-analysis
Xinyi Lin MD, Yan Wu PhD, Hong Huang MD, Ruihan Peng, FuHua Huang MD, Lvrong Hong MD, WenZhuan Chen MD

Decreased Health Care Expenditure and Average Length of Therapy With Facilitated Transition
Discharge Program for Patients Receiving Negative Pressure Wound Therapy Leila Boti Laura Soloway Deb Myers Dinu Pillai Javad Zabihi
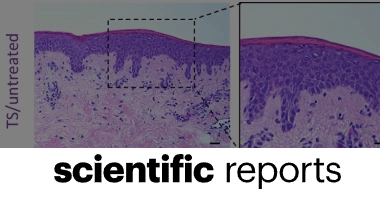
Fig latex inhibits the growth of pathogenic bacteria invading human diabetic wounds and accelerates wound closure in diabetic mice
Mohamed Salah, Gamal Badr, Helal F. Hetta, Walaa A. Khalifa & Ahmed A. Shoreit
Venturis Therapeutics, Inc. Announces Affiliation With David G. Armstrong DPM MD PhD Leading Authority on Diabetic Foot
David G. Armstrong, DPM, PhD, is an internationally recognized leader in the field of podiatric surgery, diabetic foot, limb preservation, and wound healing.
DALLAS, TX, USA, June 12, 2023/EINPresswire.com/ — Venturis Therapeutics, Inc. (“VT” or the “Company”) announces that David G Armstrong, DPM MD PhD will assume the role of senior academic advisor for the surgeon board. This agreement was consummated following a review of the Company’s scientific and commercial opportunities.
Armstrong is the foremost expert in diabetic limb preservation and is recognized worldwide with a huge following through his prodigious authorship and lectures on diabetic limb salvage. This affiliation will further enhance the diabetic wound program and clinical research trials being undertaken by VT, and will improve the visibility of the Company dramatically among all those in the greater biotech wound healing community.
ABOUT VENTURIS THERAPEUTICS
Venturis Therapeutics, Inc. is a biopharmaceutical company developing protein drug candidates to address diseases such as severe coronary heart disease, diabetic wounds, peripheral artery disease, erectile dysfunction, stroke, and spinal disk disease. The active pharmaceutical ingredient (“API”) in our drug candidates is FGF-1, a human protein that stimulates the growth of new blood vessels, thereby increasing the blood supply to ischemic organs and tissues.
FORWARD LOOKING STATEMENTS
This news release contains forward-looking statements that involve risks and uncertainties. Actual results and outcomes may differ materially from those discussed or anticipated. For example, statements regarding expectations for new research, progress with clinical trials or future business initiatives are forward looking statements. Factors that might affect actual outcomes include, but are not limited to, FDA approval of VT drug candidates, market acceptance of VT products by customers, new developments in the industry, future revenues, future expenses, future margins, cash usage, and financial performance. Additionally, until VT is cash flow positive from operations, the Company is dependent upon raising capital to fund its operations and meet its obligations as they come due. There can be no assurance that VT will be able to raise the necessary capital when needed.
Amy Gordon
Venturis Therapeutics Inc
+1 972-904-2029
email us here
Dr. David G Armstrong The 18th Malvern Diabetic Foot Conference Future of Wound Treatment

You Put WHAT on Your Wound?
by Caroline Fife, M.D.


Combining SNAPs with antibiotics shows enhanced synergistic efficacy against S. aureus and P. aeruginosa biofilms
Ramón Garcia Maset, Alexia Hapeshi, John Lapage, Niamh Harrington, Jenny Littler, Sébastien Perrier & Freya Harrison

Accelerated Wound Healing in Diabetic Rat by miRNA-185-5p and Its Anti-Inflammatory Activity
Kui-Xiang Wang,1 Li-Li Zhao,1 Ling-Tao Zheng,2 Li-Bin Meng,1 Liang Jin,3 Long-Jun Zhang,4 Fan-Lei Kong,1 Fang Liang
Novel 3D-printed wound dressing could enhance healing of burn patients
This polymer-based dressing might also be utilized to deliver chemotherapy in cancer treatment. Mrigakshi Dixit
Digital Innovation Initiative Aims to Reduce Amputations
RALEIGH, N.C.–(BUSINESS WIRE)–In the United States alone, a leg is amputated every two minutes. As announced at last week’s New Cardiovascular Horizons (NCVH) Annual Conference, this confronting statistic is being met head on by a digital innovation initiative referred to as SL2 for “Saving Limbs. Savings Lives.” Enrollment in SL2 is now open to all members of the American Podiatric Medical Association (APMA), the country’s largest non-profit organization dedicated to advancing the practice of foot and ankle medicine.
APMA members will have access to CarePICS, a software application purpose built to support best practices in wound care, including electronic consults and electronic referrals for optimal collaboration with peers in the vascular community. As an added benefit, many of the activities facilitated within CarePICS are eligible for reimbursement. The application may be used via mobile phones, desktop computers and iPads/tablets. It does not require any special devices.
For more information on SL2 and to enroll, visit https://carepics.com/apma/sl2.
The nationwide launch of SL2 is considered Phase 2 of the digital innovation initiative, with Phase 1 having drawn to a close at the end of May 2023, following a successful six-month pilot in Florida and Texas. Phase 2 will run for 12 months, through June 2023, at which time the potential for a permanent program will be evaluated.
The steadily increasing rate of lower extremity amputations in the United States – an estimated 60% deemed preventable – is shown to be the result of four key variables: (1) imprecise wound assessment and measurement (using manual tools), (2) inadequate and inconsistent wound documentation, (3) suboptimal patient follow-up and communication, and (4) fragmented coordination between providers who encounter wounds, such as podiatrists, and vascular specialists who treat associated medical conditions, mainly peripheral arterial disease (PAD) and critical limb ischemia (CLI). SL2 is positioned to combat these variables through a combination of digital tools, educational courses, data analysis and member support.
“When we look broadly at the medical histories of patients who have undergone lower extremity amputations, the evidence reveals that only about half have ever had a vascular evaluation or were referred to a vascular specialist. Their condition simply progressed to a stage where the limb could not be salvaged,” says Dr. Timothy Yates of Palm Vascular Centers, who participated in Phase 1 of SL2. “This is exactly the scenario SL2 is helping avoid. Using the CarePICS app, podiatrists can quickly and easily request an electronic consult with a vascular specialist, then convert it to an electronic referral when it’s indicated that the patient needs a vascular evaluation.”
“The CarePICS app has been a game changer for our wound care practice,” says Dr. Eric Lullove of West Boca Center for Wound Care, who also participated in Phase 1 of SL2. “Not only can we achieve precision and efficiency in our wound measurement and documentation, our patients can message with us, they can upload images, even participate in televisits. It is also possible to order wound dressings and cellular tissue products through the app.”
SL2 is governed by an advisory panel of physicians with expertise in podiatric and vascular medicine (in alphabetical order):
- David B. Alper, DPM
Member of Board of Trustees, American Podiatric Medical Association
Board Member, American Diabetes Association – Northeast Region
- Vinod A. Chainani, MD, FACC, FSCAI
Interventional Cardiologist and Endovascular Specialist, Palm Vascular Centers
- Paramjit “Romi” Chopra, MD
Founder, Chairman and CEO, MIMIT Health
- Vickie R. Driver, DPM, MS, FACFAS, FAAWC
Chair, Wound Care Collaborative Community
Professor of Medical Education, University of Virginia School of Medicine
- Matthew G. Garoufalis, DPM, FASPS, FACPM, CWSD, FFPM, RCPS(Glasg), FRSM
President, Professional Foot Care Specialists, PC
Co-Chair, Alliance of Wound Care Stakeholders
Past President, American Podiatric Medical Association
- Alton R. Johnson, Jr., DPM, DABPM, FACPM, FASPS, CWSP
Attending Physician, Clinical Assistant Professor and RISE Innovation Fellow, University of Michigan
Chair of Board of Directors, American Society of Podiatric Surgeons
- M. Laiq Raja, MD, FACC, FSCAI
Director and Co-Founder, Pulse Amputation Prevention Centers
Interventional Cardiologist and Limb Salvage Specialist, El Paso Cardiology Associates, P.A.
Medical Director of Cardiology and Critical Limb Ischemia Program, The Hospitals of Providence Memorial Campus
The SL2 advisory panel is directed by Christopher K. Bromley, DPM, FACFAS, Chief Medical Officer of CarePICS and Adjunct Professor at Kent State University College of Podiatric Medicine.
About CarePICS
CarePICS is a health tech company on a mission to save limbs and save lives through efficient, high-value digital tools purpose built to foster best practices in wound care, including precision measurement, compliant documentation, patient self-reporting, and streamlined collaboration among all providers in the continuum. CarePICS may be used in podiatry, vascular medicine, primary care, endocrinology, cardiology, oncology, plastic surgery, dermatology, geriatrics and myriad other clinical disciplines. The software platform serves as the backbone of two nationwide programs aimed at reducing preventable lower extremity amputations: SL2 and Collaborate4Wounds. CarePICS was founded by Paul Schubert and Terry Williams, both industry veterans of wound care and healthcare technology innovation. For more information, visit www.carepics.com.
Contacts
Joy Efron, Principal
Kibit Marketing
joy@kibitmarketing.com
The Most Positive Thing to Happen to Negative Pressure Wound Therapy
NEXA NPWT System Launched in the USA
OCEANSIDE, Calif., June 7, 2023 /PRNewswire/ — AOTI Inc, the global leader in multi-modality topical wound oxygen, announced exciting news from ongoing WOCNext conference in Las Vegas, Nevada, where their game changing NEXA Negative Pressure Wound Therapy (NPWT) system made its official USA debut.
The unique NEXA NPWT system is an incredibly flexible platform that is simple to use, silent, portable and affordable. NEXA seamlessly combines the simplicity of disposable NPWT with the performance features of more traditional durable NPWT technology platforms.
Dr. Mike Griffiths, CEO and President of AOTI commented; “Releasing the innovative NEXA NPWT platform in the USA is a major milestone for the company that will allow clinicians, payers, and patients alike to experience much improved performance at significantly lower cost. With NEXA, we have reimagined how NPWT should function.
Its addition to our portfolio only further enhances our mission of helping all people with chronic conditions get back to living their lives to the fullest.”
AOTI’s unique NEXA NPWT and Topical Wound Oxygen (TWO2) therapy are unlike any other treatment approaches. NEXA provided unrivaled flexibility and performance in a portable NPWT system. TWO2 is the only device that provides a multimodality treatment, combining higher pressure oxygen delivery with non-contact cyclical compression and humidity, in a therapeutic applied by the patient at home. This patented approach has been demonstrated in numerous Randomized Controlled Trial (RCT) and Real World Evidence (RWE) studies to not only heal chronic wounds at a far higher rate, but perhaps more importantly, keep them closed longer term, thereby reducing unnecessary hospitalizations and amputations.1, 2
1 Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers; The TWO2 Study. Robert G. Frykberg et al, Diabetics Care 2020; 43:616-624. https://doi.org/10.2337/dc19-0476.
2 Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes; Jessica Izhakoff Yellin, et al; Advances in Wound Care 2022; http://doi.org/10.1089/wound.2021.0118
About AOTI
AOTI is a privately-owned company based in Oceanside, California USA and Galway, Ireland that provides innovative solutions to resolve severe and chronic wounds worldwide. Our mission is to help all people with chronic conditions get back to living their lives to the fullest. We do this by enhancing access to care, improving quality of life and advancing health equity.
Our products reduce healthcare costs and improve the quality of life for patients with these debilitating conditions. Our patented Topical Wound Oxygen (TWO2) at home therapy is clinically proven to deliver Sustained Wound Healing that reduces both Amputations and Hospitalizations, So Life Can Get Back to Normal.
For more information see: www.aotinc.net
Contact:
Dr. Mike Griffiths
CEO & President
360322@email4pr.com
(760) 672 1920
SOURCE AOTI Inc.

Results of Neuropathy Screening Test for Lower Limb Amputees With Diabetes Mellitus and Their Prosthetic Rehabilitation
A Cross-Sectional Study Yohei Tanaka • Takaaki Ueno


Challenges faced by individuals living with foot and wound disease.
Foot and wound chronic disease is characterized by persistent ulcers, sores, and infections that primarily affect the feet. it is a condition that causes pain, affects mobility issues, and even long-term disability if left untreated. On today’s health beat, we delve deeper into the challenges faced by individuals living with foot and wound disease.

Predictive Factors for Lower Limb Amputation in Type 2 Diabetics
Sawsen Nouira • Taïeb Ach • Foued Bellazreg • Asma Ben Abdelkrim

Placental Based Allografts: From Womb to Wound
In this webcast, Dr. Vincent W. Li reviews the biology of placental based allografts and the components relevant to wound healing, regeneration, and tissue repair. From May 2, 2019
To learn more about how amnio allografts can benefit your patients with chronic wounds as well as reimbursement information schedule a meeting at support@AmnioSource.com or call (336) 223-5050

Topical Insulin as an Add-On Therapy for Leg Ulcer: A Case Report
Yusuf Can Edek • Elif Çalışkan Güneş • Hale Nur Ertugay Aral • Esra Adışen • Ahmet Burhan Aksakal


DDI tackles diabetic foot complications
Dasman Diabetes Institute (DDI), established by the Kuwait Foundation for the Advancement of Sciences, held recently its specialized three-day course on the prevention and management of diabetic foot complications, in collaboration with the Primary Health Care Department at the Ministry of Health who assisted in coordinating this course. The program was held at the Institute…
Sonoma Pharmaceuticals Introduces Next Generation Solution for Pulse Lavage Irrigation in the European Union
BOULDER, CO / ACCESSWIRE / June 8, 2023 / Sonoma Pharmaceuticals, Inc. (Nasdaq:SNOA), a global healthcare leader developing and producing patented Microcyn® technology based stabilized hypochlorous acid (HOCl) products for a wide range of applications, including wound care, dermatology, and eye, oral and nasal care, today announced a new application for intraoperative pulse lavage irrigation treatment, which can replace commonly used IV bags in a variety of surgical procedures.
Sonoma developed this new application of its wound care technology in response to an unmet need for a non-toxic irrigation solution that can prevent infection and improve healing time. The intraoperative pulse lavage container is designed to be used in combination with a pulse lavage irrigation device, or flush gun, for abdominal, laparoscopic, orthopedic, and periprosthetic procedures. This product replaces commonly used non-antimicrobial saline and aggressive rinsing solutions with safe and effective Microcyn® Technology. Microcyn® Technology assists in the reduction of microorganisms, is non-toxic, and has regenerative properties, making it critical in preventing infection and promoting wound healing. Sonoma’s pulse lavage container is also cost competitive with IV bags, the current standard of care.
Sonoma developed the intraoperative pulse lavage irrigation treatment in close collaboration with the medical community and Sonoma’s existing distribution partners in Europe and expects this new application will be met with wide acceptance. Sonoma is now accepting orders for the pulse lavage irrigation treatment solution, which is expected to be ready for commercial use in Europe in September 2023. Sonoma anticipates commercial launch in the U.S. in 2024.
“Sonoma continues to lead in the innovation of products that improve outcomes for people with wounds or injuries or who are needing surgery. We continue to see increased demand for our wound care products in Europe, and we are excited to expand our offerings to include this next generation irrigation solution to help people heal faster following surgery,” said Amy Trombly, CEO of Sonoma Pharmaceuticals.
For more information, or to pre-order our pulse lavage irrigation treatment solution in Europe, please contact info.europe@sonomapharma.com.
About Sonoma Pharmaceuticals, Inc.
Sonoma Pharmaceuticals is a global healthcare leader for developing and producing stabilized hypochlorous acid (HOCl) products for a wide range of applications, including wound care, eye care, nasal care, oral care, dermatological conditions, animal health care and non-toxic disinfectants. The company’s products reduce infections, itch, pain, scarring and harmful inflammatory responses in a safe and effective manner. In-vitro and clinical studies of hypochlorous acid (HOCl) show it to have impressive antipruritic, antimicrobial, antiviral and anti-inflammatory properties. Sonoma’s stabilized HOCl immediately relieves itch and pain, kills pathogens and breaks down biofilm, does not sting or irritate skin, and oxygenates the cells in the area treated, assisting the body in its natural healing process. The company’s products are sold either directly or via partners in 55 countries worldwide and the company actively seeks new distribution partners. The company’s principal office is in Boulder, Colorado, with manufacturing operations in Guadalajara, Mexico. European marketing and sales are headquartered in Roermond, Netherlands. More information can be found at www.sonomapharma.com. For partnership opportunities, please contact busdev@sonomapharma.com.
Forward-Looking Statements
Except for historical information herein, matters set forth in this press release are forward-looking within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including statements about the commercial and technology progress and future financial performance of Sonoma Pharmaceuticals, Inc. and its subsidiaries (the “company”). These forward-looking statements are identified by the use of words such as “continue,” “develop,” “anticipate,” “expect” and “expand,” among others. Forward-looking statements in this press release are subject to certain risks and uncertainties inherent in the company’s business that could cause actual results to vary, including such risks that regulatory clinical and guideline developments may change, scientific data may not be sufficient to meet regulatory standards or receipt of required regulatory clearances or approvals, clinical results may not be replicated in actual patient settings, protection offered by the company’s patents and patent applications may be challenged, invalidated or circumvented by its competitors, the available market for the company’s products will not be as large as expected, the company’s products will not be able to penetrate one or more targeted markets, revenues will not be sufficient to meet the company’s cash needs, fund further development, as well as uncertainties relative to the COVID-19 pandemic and economic development, varying product formulations and a multitude of diverse regulatory and marketing requirements in different countries and municipalities, and other risks detailed from time to time in the company’s filings with the Securities and Exchange Commission. The company disclaims any obligation to update these forward-looking statements, except as required by law.
Sonoma Pharmaceuticals™ and Microcyn® are trademarks or registered trademarks of Sonoma Pharmaceuticals, Inc. All other trademarks and service marks are the property of their respective owners.
Media and Investor Contact:
Sonoma Pharmaceuticals, Inc.
SOURCE: Sonoma Pharmaceuticals, Inc.

New project applies digital health tools to curb amputations
The “Saving Limbs, Saving Lives” campaign aims to give healthcare providers access to a digital health platform that offers care management resources and a virtual connection to specialists
Global Innovation in Wound Care Summit: Part 1 Debridement Masterclass
Come and learn all about debridement from our global wound care experts!

Wirelessly-powered ‘smart bandage’ could provide drug-free wound care
A new generation of wirelessly-powered, environmentally-friendly ‘smart bandages’ could help patients with non-healing wounds avoid infections, scientists say. The bandage could help improve the quality of life of people who live with chronic non-healing wounds, which currently frequently require painful cleaning and treatment. Non-healing wounds can be a side effect of certain medications or health factors like diabetes, cancer or damaged blood vessels. The smart bandage is built on technology initially developed by Dr Mahmoud Wagih of the James Watt School of Engineering and his colleagues from the University of Southampton. The research is showcased in a second paper, recently published in IEEE Transactions on Industrial Electronics. The paper demonstrates the first use of magnetic-resonant wireless power transfer to provide electricity to standard textiles using embroidery or screen printing – a feature which helped to make the smart bandage possible. In this case, the power was supplied to a newly-developed flexible electronic resistor made from silver and carbon which was printed into a textile surface to act as a wearable heating element. The system was capable of being heated to up to 60◦C while separated from the transmitter by 2cm at an efficiency exceeding 50%.
Swift Medical Announces Proven Outcomes Across 20 Million Wound Assessments
Swift’s AI-powered wound imaging technology shown to improve quality of care and costs to manage complex patients
June 01, 2023 09:00 AM Eastern Daylight Time
TORONTO–(BUSINESS WIRE)–Swift Medical, a digital health technology company focused on improving clinical and economic outcomes in wound care, today announced proven outcomes from more than 20 million wound assessments captured with Swift’s leading, AI-powered wound care platform. Deployed in nearly 4,000 healthcare facilities across North America, Swift’s technology has been shown to speed up wound healing by 37%, reduce wound prevalence and hospitalizations by 35% and 14%, respectively, and reduce hospital length of stay by 62%.
“We are proud to share the incredible impact our technology is having on the millions of patients living with wounds – one of today’s most expensive and overlooked threats to patients and our overall healthcare system”
Tweet this
“We are proud to share the incredible impact our technology is having on the millions of patients living with wounds – one of today’s most expensive and overlooked threats to patients and our overall healthcare system,” said Brian Litten, CEO of Swift Medical. “These outcomes demonstrate the impact of having the most powerful wound image dataset in the world and its ability to deliver high quality wound care with reduced costs.”
The Swift Skin & Wound mobile application enables any mobile device to be equipped with AI-powered imaging capabilities to capture clinically validated, high-precision 3D images, measurements, and other clinical data. The imaging captured at the point of care enables real-time, at-risk patient monitoring, proactive decision-making, and remote wound consultations, reducing the time and cost spent evaluating wounds to create a more efficient wound care experience for both clinicians and patients.
Today, more people suffer from chronic wounds than those with breast cancer, colon cancer, lung cancer, and leukemia combined. Despite this costly, growing problem, the current standard of care is outdated and highly inaccurate. Clinicians typically receive less than 10 hours of formal education and rely on paper rulers for measurements and cotton swabs for depth assessment. This inefficient and ineffective approach is both painful for patients and leads to poor diagnostic accuracy, prolonged healing, and inappropriate selection of therapies, putting patients at greater risk for readmission, longer lengths of stay and higher care costs.
With its advanced analytics and proven outcomes, Swift is now poised to partner with health plans and risk-bearing providers to deliver high quality, value-based wound care at scale.
About Swift Medical
Swift Medical is the global leader in digital wound care. We are headquartered in Toronto, with operations expanding across the U.S. and Canada. Our mission is to make empathy-driven wound care ubiquitous through AI-powered diagnostic technology. We are the trusted wound technology partner of more than 4,000 healthcare facilities in North America across the continuum of care. Our solutions empower healthcare providers to deliver standardized, accessible and equitable wound care for every patient – with advanced, high-precision imaging, compliant documentation, clinical analytics and remote care. To learn more about Swift Medical, visit us at www.swiftmedical.com.
Contacts
Media
David Mannion
416-303-8020
david.mannion@swiftmedical.com

CORE Principles of Managing Venous Leg Ulcers
Windy Cole, DPM, CWSP, FACCWS

Flesh Fly Maggot-Infected Abscess in a Burn Victim Patient in the United States Without a History
of International Travel: A Case Report Andy Aleman Espino • Erik Aleman Espino • Claudia Aleman Oliva • Gustavo Huaman • Ricardo Castrellon

Histologics Future of Debridement Wound Masterclass
Comprehensive review of removing tissue from wounds for diagnostic or therapeutic (debridement/wound hygiene) with evidence and case examples. “Future of Debridement” segment by Dr. Neal Lonky and Dr. Traci Kimball ; May 31st, 2023 on Wound Masterclass


So, You Want To Be A Wound Care Specialist?
Michael King, Medical Director, Upperline Health

DIONYSIUS trial: “Does increasing oxygen nurture your symptomatic ischaemic ulcer sufficiently?”
Study protocol for an international multicentre randomised trial Robin Brouwer, Rowan van der Peet, Rigo Hoencamp, Mark Koelemay, Susan van Dieren, Rob van Hulst, Dirk Ubbink


Superficial Surgical Site Infections in Primary Total Joint Arthroplasty: A Retrospective Analysis of Topical Anti-Biofilm Therapy
Joseph P. Kelly • Andrew S. Bae • Jacob Taunton • Achraf Jardaly • Robert M. Harris

How to Tell If the Wound Is Healing or Infected?
Ieva Aleknaitė-Dambrauskienė, PhD


Helping Your Patients be Encouraged, Not Malnourished
NANCY COLLINS

Dysphagia and Wounds: Making the Connection
Nancy Collins, PhD, RDN, LD, NWCC, FAND

Expressing the Patient’s Experience in Wound Care
Brian McCurdy, the Managing Editor of Today’s Wound Clinic, and I talked about a project we are working on to elevate the patient’s voice in wound care Caroline Fife, M.D.


Developing A Wound Care Culture
David Navazio


Overcoming biological barriers to improve treatment of a Staphylococcus aureus wound infection
Virginie Papadopoulou, Ashelyn E. Sidders, Kuan-Yi Lu, Amanda Z. Velez, Phillip G. Durham, Duyen T. Bui, Michelle Angeles-Solano, Paul A. Dayton, Sarah E. Rowe



IR-MED Announces Demonstration of AI-driven Analysis Tech Platform
IR-Med’s PressureSafe Noninvasive Wound Care Device to be Demonstrated at Northwell Health’s 10th Annual Shining the Light on Wound Care Symposium

A new algorithm for the management of diabetic foot ulcer: recommendations from Central and Eastern Europe
Robert Bem, Paul Chadwick, Ivan Cvjetko, Miroslav Koliba, Zoltan Kokeny, Przemyslaw Lipinski, Beata Mrozikiewicz-Rakowska, Istvan Rozsos, Adam Wegrzynowski
Three genes expressed in relation to lipid metabolism considered as potential biomarkers
for the diagnosis and treatment of diabetic peripheral neuropathy Ye Yang & Qin Wang

New Expert Guidelines from IWGDF Highlight the Growing International Recognition of Topical
Oxygen Therapy for Diabetic Foot Ulcers
Evidence-based recommendations support the effective use of TOT in diabetic foot ulcer management, revolutionizing treatment approaches worldwide
CAMBRIDGE, England, May 17, 2023 /PRNewswire/ — NATROX® Wound Care, a leading innovator in wound care technology, proudly announces the release of newly published expert recommendations on the use of topical oxygen therapy for wound healing1. The updated guidelines endorse topical oxygen therapy (TOT) as an adjunct therapy in the treatment of diabetic foot ulcers (DFUs) 1. With its endorsement by leading experts, this ground-breaking therapy is poised to transform the lives of millions of people worldwide, offering renewed hope for effective healing and improved quality of life.
New IWDGF Guidelines
The International Working Group on the Diabetic Foot (IWGDF) has just published its 2023 Guidelines. This set of recommended DFU interventions, developed by a panel of renowned experts, serves as a trusted resource for healthcare professionals worldwide.
Notably, among the 29 recommendations highlighted, TOT gained recognition as an accepted intervention when treating non-healing DFUs. “Consider the use of topical oxygen as an adjunct therapy to standard of care for wound healing in people with diabetes-related foot ulcers where standard of care alone has failed and resources exist to support this intervention1.” With its inclusion in the IWGDF guidelines, topical oxygen therapy emerges as a vital tool, poised to revolutionize the management and healing of foot ulcers in individuals with diabetes.
In addition, the guidelines note that “evidence on topical oxygen has substantially expanded in the last four years with several new RCTs with a total of ten included in the systematic review for these guidelines (References 100-109) 1“, which includes an RCT study published in 2021² which compared the healing effects of using standard care against a combination of standard care plus NATROX® O₂ topical oxygen therapy. In the study, patients completing the therapy experienced 71% greater healing rates² and 73% greater reduction in wound size² with NATROX® O₂.
Experts recommend updating algorithms to include TOT
In the Journal of Wound Care, experts reached a “clear consensus that adjunctive treatments with a solid evidence base, including NPWT and TOT, must be included“3 in each of the four proposed regional guidelines. Most notably, the experts agreed that “all hard-to-heal wounds are likely to benefit from TOT³.”
TOT received “A grade” from the American Diabetes Association
The American Diabetes Association recently released its “Standards of Care in Diabetes⁴” which not only recommended TOT as an adjunctive therapy for chronic DFUs, but also gave it an “A grade” based on the quality of evidence⁴. The newly published recommendations acknowledge the remarkable potential of TOT⁴.
According to Dr. Windy Cole, DPM, CWSP, FAPWH, FACCWS, renowned authority in podiatric medicine and dedicated wound care advocate for over two decades, “The evidence supporting the efficacy of TOT is now undeniable. It is imperative that healthcare professionals embrace this innovative yet simple approach to achieve improved healing outcomes.” After witnessing the positive impact topical oxygen therapy can have on healing DFUs in her own clinic, Windy recently joined the NATROX® team as Director of Global Medical Affairs to further advocate for the integration of topical oxygen therapy in the treatment pathway for chronic wounds.
NATROX® Wound Care CEO, Craig Kennedy, expressed great enthusiasm regarding the recognition and international acceptance of topical oxygen therapy, stating, “We’re delighted that topical oxygen therapy continues to gain international recognition, cementing its status as a game-changing treatment in wound care. The inclusion of topical oxygen in the IWGDF Guidelines further validates our mission to transform the quality of life for patients suffering from chronic wounds, particularly those with diabetic foot ulcers.”
What is NATROX® O₂ Topical Oxygen Therapy NATROX® Wound Care manufactures an award-winning5,6,7 topical oxygen therapy device known as NATROX® O₂. The compact, wearable device generates and delivers a continuous flow of oxygen directly to the wound bed to promote accelerated healing and foster a healthy wound environment. Its non-invasive nature, coupled with its remarkable effectiveness², offers a significant advancement in chronic wound treatment, even allowing patients to be treated from the comfort of home.
To learn about NATROX® O₂ and request a demo, visit: https://bit.ly/NO2therapy
About NATROX® Wound Care
NATROX® Wound Care is an Inotec AMD brand. The specialist wound care company based in Cambridge, England was formed specifically to introduce new technologies to healthcare professionals around the world to promote faster and better healing to patients. The company’s flagship product, NATROX® O₂, is positioned to become an integral part of global wound care treatment regimes in the coming years. To learn more, explore the website: natroxwoundcare.com.
See the references: https://bit.ly/nwc-iwgdf-guidelines
Media Contact:
NATROX® Wound Care
Nancy Stahulak
VP Global Marketing
marketing@natroxwoundcare.com
+1 (888) 354 9772
Zinc chloride is effective as an antibiotic in biofilm prevention following septoplasty
Noa Noach, Eran Lavy, Ram Reifen, Michael Friedman, David Kirmayer, Einat Zelinger, Amit Ritter, Dan Yaniv & Ella Reifen

Antibiotic treatment can exacerbate biofilm-associated infection by promoting quorum cheater development
Lei He, Huiying Lv, Yanan Wang, Feng Jiang, Qian Liu, Feiyang Zhang, Hua Wang, Hao Shen, Michael Otto & Min Li
2 Minute Medicine Rewind May 22, 2023
Patients with diabetic foot ulcers randomized to receive an application of topical esmolol hydrochloride in addition to standard care were observed to have an improved rate of wound closure at 12 weeks

Breaking Through Bacterial Barriers in Chronic Treatment-Resistant Wounds
Researchers in the UNC School of Medicine’s Department of Microbiology and Immunology and the UNC-NC State Joint Department of Biomedical Engineering have developed a new strategy to improve drug-delivery into chronic wounds infections Sarah Rowe-Conlon, PhD Paul Dayton, PhD

Topical Oxygen Therapy Awarded Positive Treatment Recommendation by the International
Working Group on the Diabetic Foot in their 2023 Diabetic Foot Ulcer Guidelines OCEANSIDE, Calif., May 15, 2023 /PRNewswire/ — AOTI Inc, the global leader in multi-modality topical wound oxygen, announced exciting news from the 9th International Symposium on the Diabetic Foot (ISDF). The ISDF is often referred to as the Olympics of the Diabetic Foot, due…

Topical Esmolol Gel Helped Heal Diabetic Foot Ulcers
by Kristen Monaco, Senior Staff Writer, MedPage Today May 15, 2023

Long-Term Follow-Up of Open Gustilo-Anderson IIIB Fractures Treated With an
Adjuvant Local Antibiotic Hydroxyapatite Bio-Composite Joshua A. Henry • Almigdad Ali • Ibrahim H. Elkhidir • Adam Reid • Jason Wong • Anand Pillai

Largest Published Real-World Wound Imaging Study Reports MolecuLight®
led to Wound Treatment Plan Changes in up to 53% Cases Results from 211 Facilities Show MolecuLight Imaging is a Valuable Toolin Improving Bacterial Infection Management TORONTO, May 23, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated, pathogenic bacterial loads in and around wounds, announced the publication of their…

Study reviews skin cancers among EB patients
by Steve Bryson, PhD


Aurealis Therapeutics receives CTA approval for AUP-16 Phase 2 RCT in Diabetic Foot Ulcer patients
Multi-center, randomized, placebo-controlled diabetic foot ulcer (DFU) Phase 2 study to start May 2023 in Italy, Germany, and Poland After completing 12 months follow-up of our DFU Phase 1 study, and 10M CHF Series A financing round to accelerate our clinical program, these are fantastic news for the company”— Juha Yrjänheikki, CEO BASEL, SWITZERLAND, May…
Microbion Corp. Presents a Poster on Pravibismane’s Activity Against Diabetic Foot Infection Patient Isolates
BOZEMAN, Mont., May 10, 2023 /PRNewswire/ – Microbion Corporation today announced that the company presented a poster focusing on pravibismane’s activity against diabetic foot ulcer infection pathogens at the 9th International Symposium on the Diabetic Foot that is currently ongoing from May 10th to 13th, 2023 at The Hague, Netherlands. The poster highlights pravibismane’s activity versus comparator antibiotics against pathogens isolated from diabetic foot infection (DFI) patients in an earlier Phase 1b clinical study. Poster Details:
Title: Broad-Spectrum, Potent Activity of Pravibismane Versus Comparators Against Diabetic Foot Ulcer Infection Patient Isolates Collected in a Phase 1b Study Presenter: Dr. Jeff Millard, CSO Poster Highlights:
"We are pleased that pravibismane demonstrated extremely potent MIC activity against clinical DFI isolates, which was in line with in vitro AST microbial pre-clinical studies," said Dr. Jeff Millard, CSO of Microbion Corp. "Diabetic foot infections are often infected by several different bacterial species concurrently, which may change over the chronicity of the wound, from predominantly aerobic to anaerobic. We believe pravibismane’s potent broad-spectrum activity is potentially a key treatment advantage since a single agent could eradicate both aerobic and anaerobic bacteria, thereby decreasing the need for multiple systemic therapies." Bacterial cultures for this study were grown from swabs collected at the wound bed at baseline visit and antimicrobial susceptibility testing (AST) was performed on isolated pathogens. Pathogen isolation and AST was performed at Investigational Health Management Associates (IHMA, IL), using the Clinical & Laboratory Standards Institute (CLSI) standard methods. Topical pravibismane has received QIDP and Fast Track drug designation from the US FDA for the adjunctive treatment of moderate and severe diabetic foot ulcer infections. Topical pravbismane is currently enrolling in a Phase 2 clinical study to further evaluate its safety and efficacy in subjects suffering from moderate infections associated with chronic diabetic foot ulcers. About Microbion
Microbion is a clinical-stage pharmaceutical company developing a new class of therapeutic compounds to improve the lives of patients with rare and serious diseases. Microbion’s lead drug candidate, pravibismane, is the first product in this new class and has multiple novel modes of action offering unique potential to address the unmet needs of chronic and severe health conditions. Topical/local pravibismane is in Phase 2 development for the treatment of chronic wounds and orthopedic infections. The Company is advancing inhaled pravibismane in Phase 1 clinical development for the treatment of chronic lung diseases, including non-tuberculous mycobacteria (NTM) and cystic fibrosis-related lung infections. Pravibismane has received backing from the Cystic Fibrosis Foundation, NIH, US DoD, and CARB-X with over $21 million in grants. The FDA has granted pravibismane with Orphan Drug, Fast Track, and QIDP designations. For more information visit: www.microbioncorp.com. Safe Harbor Statement
Certain of the statements made in this press release are forward-looking, such as those, among others, relating to the success of clinical development of pravibismane and preparation for potential commercialization. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: our ability to enroll patients in our clinical trials at the pace that we project; the size and growth of the potential markets for pravibismane or any future product candidates and our ability to serve those markets; our ability to obtain and maintain regulatory approval of pravibismane or any future product candidates; and our expectations regarding the potential safety, efficacy or clinical utility of pravibismane or any future product candidates. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Microbion Corporation disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. SOURCE Microbion Corporation |

New Bandage Changes Color in Case of Infection
The novel dressing reduces risk of complications and will contribute to the fight against antibiotic resistance.

Hidden in Plain Sight: Understanding Lymphedema and Lymphatic Function in Wound and Tissue Healing
Heather L. Barnhart, PT, PhD, CWS, AWCC, CLT-LANA, CLWT, CORE

Amputations in Pyoderma Gangrenosum (Need Your Help Again!)
by Caroline Fife, M.D.

Topical vanadate improves tensile strength and alters collagen organisation of excisional wounds in a mouse model
Hendrik Lintel BS, Darren B. Abbas MD, Duncan J. Mackay MD, MBA, Michelle Griffin MBChB, PhD, Christopher V. Lavin MD, Charlotte E. Berry BS, Nicholas J. Guardino BS, Jason L. Guo PhD, Arash Momeni MD, Donald R. Mackay MD, DDS, Michael T. Longaker MD, MBA, Derrick C. Wan MD

A Comprehensive Review of the Application of Nanoparticles in Diabetic Wound Healing:
Therapeutic Potential and Future Perspectives


A Novel Bifunctional Nanoplatform with Aggregation-Induced Emission Property for
Efficient Photodynamic Killing of Bacteria and Wound Healing Authors Hou B, Yang F, Hu C, Liu C, Xiao X, Chen Y, Huang X, Xie S

Clinical utility of methicillin-resistant Staphylococcus aureus nasal PCR to streamline antimicrobial
use in treatment of diabetic foot infection with or without osteomyelitis Gaielle Harb, Teri Hopkins, Linda Yang, Kathleen Morneau, Jose Cadena-Zuluaga, Elizabeth Walter & Christopher Frei

Antibiotic-loaded bone substitutes therapy in the management of the moderate to severe diabetic foot infection:
A meta-analysis Xinyi Lin MD, Yan Wu PhD, Hong Huang MD, Ruihan Peng, FuHua Huang MD, Lvrong Hong MD, WenZhuan Chen MD

Fig latex inhibits the growth of pathogenic bacteria invading human diabetic wounds and accelerates wound closure in diabetic mice
Mohamed Salah, Gamal Badr, Helal F. Hetta, Walaa A. Khalifa & Ahmed A. Shoreit

Decreased Health Care Expenditure and Average Length of Therapy With Facilitated
Transition Discharge Program for Patients Receiving Negative Pressure Wound Therapy Leila Boti Laura Soloway Deb Myers Dinu Pillai Javad Zabihi

Chitosan Sponges Are Associated With Higher Rates of Wound Complications Compared to Calcium Sulfate Beads
Kelsey McKee • Joseph Easton • Brian Mullis • Ivan Hadad

A chronic wound model to investigate skin cellular senescence
Saranya P. Wyles, Parisa Dashti, Tamar Pirtskhalava, Burak Tekin, Christina Inman, Lilian Sales Gomez, Anthony B. Lagnado, Larissa Prata, Diana Jurk, João F. Passos, Tamar Tchkonia, and James L. Kirkland from Mayo Clinic in Rochester, Minnesota

CūtisCare Launches Third Annual Hyperbaric Aware™ National Campaign To Elevate Awareness Of Hyperbaric Oxygen Therapy
BOCA RATON, Fla., May 1, 2023 /PRNewswire/ — Hyperbaric Awareness USA™, a CutisCare initiative, has designated May Hyperbaric Awareness Month and is proud to announce the launch of the third annual Hyperbaric Aware™ national campaign to elevate awareness of hyperbaric oxygen therapy (HBOT). CutisCare launches a Hyperbaric Awareness USA campaign to elevate the awareness of hyperbaric oxygen…
Pulcherriminic acid modulates iron availability and protects against oxidative stress during microbial interactions
Vincent Charron-Lamoureux, Lounès Haroune, Maude Pomerleau, Léo Hall, Frédéric Orban, Julie Leroux, Adrien Rizzi, Jean-Sébastien Bourassa, Nicolas Fontaine, Élodie V. d’Astous, Philippe Dauphin-Ducharme, Claude Y. Legault, Jean-Philippe Bellenger & Pascale B. Beauregard

MolecuLight Featured in Unprecedented 24 Presentations and Posters at European
Wound Management Association (EWMA) 2023 Annual Conference Wide-Spread Clinical Evidence using the MolecuLight Imaging Platform Reveals its Significant Global Adoption and Proven Utility in Wound Care TORONTO and MILAN, May 3, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that detects and locates elevated bacterial loads in wounds, announces that its MolecuLight wound imaging platform is featured in an unprecedented…




Treatment of Pyoderma Gangrenosum With Mycophenolate and Hyperbaric Oxygen Therapy: A Case Report and Literature Review
Subo Dey • Nirali Sanghavi • Amy Wasserman • Kausik Kar


Two 3M Veraflo Therapy dressings receive first-ever FDA clearance for hydromechanical removal of non-viable tissue
Innovative therapy shown to help promote healing and reduce the need for surgical debridements creating potential savings for health care systems. ST. PAUL, Minn., April 27, 2023 /PRNewswire/ — 3M Health Care’s innovative 3M™ Veraflo™ Therapy, with both 3M™ Veraflo™ Cleanse Choice Complete™ Dressing and 3M™ V.A.C. Veraflo Cleanse Choice™ Dressing, received the first-ever Food and Drug Administration (FDA)…

Are You Seeing Drug-Induced Ulcerations? Anyone Managing Xylazine-Related Ulcerations?
by Caroline Fife, M.D.


Diabetic Foot Disease: Latest Treatment Options
By Natalie Sainz

Venous, Arterial, and Neuropathic Leg Ulcers With Emphasis on the Geriatric Population
Harvey N. Mayrovitz • Summer Wong • Camilla Mancuso

Nevro Announces Enrollment of First Patient in PDN Sensory Study
First Randomized Controlled Trial Specifically Powered to Assess Restoration of Neurological Function in Patients with Intractable Painful Diabetic Neuropathy Breakthrough Device Designation Provides Expedited Review for Marketing Application to Expand Nevro’s FDA Labeling REDWOOD CITY, Calif., April 24, 2023 /PRNewswire/ — Nevro Corp. (NYSE: NVRO), a global medical device company that is delivering comprehensive, life-changing solutions for the treatment…


The Pluses and Minuses of Adding More Services
— Should our practice add wound care and other offerings in a time of specialist shortages? by Fred Pelzman, MD, Contributing Writer, MedPage Today April 24, 2023

Disposable Slippers Enhanced with Medical Wound Care Technology
NEWS PROVIDED BY I3 BioMedical Inc. April 25, 2023, 11:00 GMT TrioMed Active Medical Technology that kills 99.9%+ of microbes in 5 minutes is now integrated in Disposable Slippers When you take your shoes off outside of your home, you expose yourself to foot infection, the Active Touch slipper provides you with the most advanced…

New Publication Reveals MolecuLight Imaging Significantly Improved
Detection of Bacterial Burden Across Patients of All Skin Tones Findings show that MolecuLight is an Objective and Equitable Diagnostic Technology Positioned to Help Level Racial Disparity in Wound Care Outcomes TORONTO, April 25, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated bacterial loads in and around wounds,…

Stimulating Wounds with Electricity for Rapid Healing
Researchers at Chalmers University of Technology in Sweden have developed a microfluidic system to test the effects of electrical stimulation in wound healing.


Preclinical performance testing of medical devices with antimicrobial effects
Hao Wang, J. Alex Chediak, Philip J. Belmont Jr, David M. Saylor & K. Scott Phillips

Smart Boots Hold Promise for Improving Adherence to Diabetic Foot Ulcer Treatment– Especially Underserved Populations
Increasing patient adherence to mechanical offloading will translate into better outcomes and a reduced number of amputations.
Nanocellulose composite wound dressings for real-time pH wound monitoring
Olof Eskilson, Elisa Zattarin, Linn Berglund Kristiina Oksman Kristina Hanna Jonathan Rakar Petter Sivlér Mårten Skog Ivana Rinklake Rozalin Shamasha Zeljana Sotra Annika Starkenberg Magnus Odén Emanuel Wiman Hazem Khalaf Torbjörn Bengtsson Johan P.E. Junker Robert Selegård Emma M. Björk Daniel Aili
Zeb1 facilitates corneal epithelial wound healing by maintaining corneal epithelial cell viability and mobility
Yingnan Zhang, Khoi K. Do, Fuhua Wang, Xiaoqin Lu, John Y. Liu, Chi Li, Brian P. Ceresa, Lijun Zhang, Douglas C. Dean & Yongqing Liu


Ten top tips: nutrition and wound healing
Liz Friedrich

Antibiotic Resistance Profile, Biofilm Formation Ability, and Virulence Factors Analysis of Three Staphylococcus spp. Isolates From Urine
Ulrich Joël Tsopmene • Yves Somo Iwewe • Isaac Mboh Eyong • Borel Ndezo Bisso • Jean Paul Dzoyem
Various effects of 11,12 EET rescue wound healing in a combined model of diabetes and ischemia
Katharina Sommer, Heike Jakob, Theresa Lettenmeier, Dirk Henrich, Jasmina Sterz, Ingo Marzi & Johannes Frank

NIH selects Northwell to join Diabetic Foot Ulcer Consortium
Northwell Health has been selected to join the Diabetic Foot Consortium as a satellite site and was awarded a grant by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Alisha Oropallo

Patients are VERA Interested in ALOE… Guest Blog by Dr. Tom Bozzuto
by Caroline Fife, M.D.

Knowledge and Practice of the Preventive and Care Methods for Diabetic Foot Among
the Caregivers of Diabetic Patients in Saudi Arabia Hassan Ali A. AlZubaidi • Ali Nori O. Alfaqih • Mohammed Hussain A. Alothayqi • Hassan Mohammed H. Alfaqih • Alaa Jameel A. Albarakati • Medhat Taha • Abdulkarim M. Alnashri


Research article network analysis of polymicrobial chronic wound infections in Masanga, Sierra Leone
Sarah Sandmann, Jonathan Vas Nunes, Martin P. Grobusch, Maxwell Sesay, Martin A. Kriegel, Julian Varghese & Frieder Schaumburg

Treatment Of Chronic Nonhealing Ulcers: How Autologous Platelet-Rich Plasma (PRP) Works
Autologous platelet-rich plasma (PRP) or autologous conditioned plasma (ACP) is widely employed in a variety of therapeutic applications. Here’s how it helps in treatment of chronic nonhealing ulcers.

Surgical Weight Loss May Reverse Neuropathy (and More True Confessions From Me)
by Caroline Fife, M.D.
Intraarterial Four-dimensional CT Angiography with Soft Tissue Perfusion Evaluation in Diabetic Feet
Pieter T. Boonen , Dimitri Aerden


Necrotic Decubitus Ulcer: DIC or Uncontrolled Bleeding Warning
Bedsores with dead tissue either get infected leading to sepsis or even disseminated intravascular coagulation that leads to uncontrolled bleeding.

MTF Biologics Unveils New Tissue Form: AlloPatch® Pliable Meshed – Acellular Human Reticular Dermal Allograft
AlloPatch® Pliable Meshed is ideal for the treatment of diabetic foot ulcers and venous leg ulcers.
REGENATIVE LABS AND DR. MICHAEL LAVOR ANNOUNCE PROSPECTIVE STUDY OF UMBILICAL CORD
TISSUE ALLOGRAFTS FOR CHARCOT FOOT

Suturing Dermatotraction Techniques in Closing Fasciotomy Wounds: A Systematic Review
Otomi O. Obuh • Ena-Jane O. Esomu • Roseline O. Sydney

Effectiveness of Topical Sucralfate in the Management of Diabetic Foot Ulcers: An Open-Labeled Randomized Study
Neha Chatterjee • Nishith M. Ekka • Mayank Mahajan • Binay Kumar • Nabu Kumar • Arquam Zia • Aravind Devarajan • Archana D. Kujur • Dipendra K. Sinha

What is the Link Between Sleep and PAD?
Mark Hinkes DPM FACFAS FAPWCA DABFAS

The Anti-Biofilm Activity and Mechanism of Apigenin-7-O-Glucoside Against Staphylococcus aureus and Escherichia coli
Ze-Jun Pei, Chengcheng Li, Wenna Dai, Zaixiang Lou, Xin Sun, Hongxin Wang, Azmat Ali Khan, Chunpeng Wan

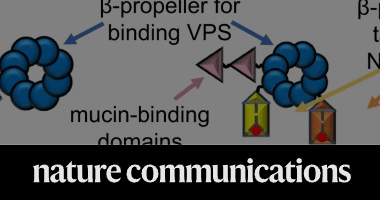
Vibrio cholerae biofilms use modular adhesins with glycan-targeting and nonspecific surface binding domains for colonization
Xin Huang, Thomas Nero, Ranjuna Weerasekera, Katherine H. Matej, Alex Hinbest, Zhaowei Jiang, Rebecca F. Lee, Longjun Wu, Cecilia Chak, Japinder Nijjer, Isabella Gibaldi, Hang Yang, Nathan Gamble, Wai-Leung Ng, Stacy A. Malaker, Kaelyn Sumigray, Rich Olson & Jing Yan

Army R&D selects local biotech firm to prevent wound infection
Press release from MLM Biologics Inc.
GAINESVILLE, Fla. – The U.S. Army Medical Research and Development Command (USAMRDC) recently awarded MLM Biologics, Inc. almost $1.5 million to develop a device to prevent infection in battlefield wounds. The device will incorporate MLM Biologic’s proprietary collagen processing and stabilization technologies.
MLM Biologics competed for the award in the battlefield treatment area, focused on preventing infection in wounds from “…complex, traumatic, penetrating injuries in a far-forward, austere environment.”
“We’re honored to bring our innovative solutions to the men and women who stand in harm’s way as our focus has always been on wound closure—not just wound management,” says CEO Chandra Nataraj.
As part of the award, MLM Biologics will develop a new medical device using its proprietary technologies combined with proven anti-inflammatory and anti-microbial components, Nataraj said. He believes it will eventually be available for civilian clinical use.
“We expect to make a difference, not only for our military heroes but eventually for everyone dealing with possible wound infection. The potential health benefits are huge,” Nataraj says.
The company maintains labs at the University of Florida’s business incubator, UF Innovate | Accelerate @ Sid Martin Biotech, where it conducts product development activities. Karl R. LaPan, UF Innovate | Accelerate director, believes MLM Biologics represents the tangible benefits incubators provide.
“MLM Biologics, and the development of a treatment to prevent wound infection, is a great example of public-private partnerships translating research into meaningful, life-changing products for America’s service members,” LaPan said.
The award is part of the Military Prototype Advancement Initiative, funded by the Department of Defense and issued through the Medical Technology Enterprise Consortium, a collaboration of industry and academia to facilitate R&D activities with USAMRDC and other government agencies.
About MLM Biologics, Inc.
MLM Biologics is a privately held medical device company specializing in the design, manufacture, and marketing of biologic medical devices. The company was founded on the modern interpretation of the Gandhian economic principle of “More for Less for Many.” It currently distributes an FDA-cleared wound care device (bio-ConneKt® Wound Matrix) out of its ISO-13485-certified facility in Gainesville, Fla. For more information, visit www.MLMBiologics.com.

An Alternate Explanation
A 48-year-old man with long-standing type 2 diabetes mellitus and chronic kidney disease presented with a 3-month history of numbness, tingling, and faint violaceous discoloration of the tips of multiple fingers and toes. Tom Alsaigh, M.D., Gurpreet Dhaliwal, M.D., Eri Fukaya, M.D., Ph.D., Nicholas J. Leeper, M.D., and Nazish Sayed, M.D., Ph.D.

Addressing the Inertia: A Holistic Approach to Diabetic Foot Evaluation
Jayshree Swain • Abhay Kumar Sahoo • Pooja A. Jadhao • S.L. Sravya • Brij R. Teli

Effect of honey dressing in the management of diabetic foot ulcers: A meta-analysis
Shaoting Li, Ting Xiao, Ning Ye, Guosheng Yang, Haiting Chen, Xia Liang, Tuodi Li, Jinying Wang, Yaozhong Peng, Yuan Li, Yanping Liu
MolecuLight Successfully Completes SOC 2® Type l Audit and Accreditation
Successful completion of recent audit demonstrates
MolecuLight’s commitment to data security
TORONTO, April 19, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated bacterial loads in and around wounds, announced that it is in compliance with SOC 2® Type I, having successfully completed its audit in accordance with the American Institute of Certified Public Accountants (AICPA) standards for Systems and Organization Controls (SOC). This enables MolecuLight to better serve the needs of its customers globally by ensuring the Company’s data management practices and organizational controls meet the highest international standards.

SOC ll (CNW Group/MolecuLight)
MolecuLight’s compliance with SOC 2® standards validates that the company has implemented controls relevant to the security, availability, and processing integrity of the systems used to process users’ data as well as the confidentiality and privacy of the information.
“Obtaining the SOC 2 certification reinforces MolecuLight’s ongoing commitment to the security, availability, and processing integrity of MolecuLight’s point-of-care fluorescence imaging technologies,” said Anil Amlani, MolecuLight’s CEO. “Our certification demonstrates our commitment and our business processes to maintain the highest level of security, privacy, availability, and confidentiality for our customers.”
As part of the process for achieving SOC 2® compliance, MolecuLight worked with PwC, an accredited auditor to review the design and operating effectiveness of the controls on its core MolecuLightDX® imaging platform against the applicable Trust Services Criteria for Security, Availability, and Confidentiality. These included the operating software of the imaging platform, how the devices manage customer and user data, as well as security policies and procedures. Over the course of the audit, MolecuLight demonstrated the ability to safely manage customers’ data and use of the platform, as well as its security controls and processes.
The MolecuLight i:X® and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 75 peer-reviewed publications involving 1,600 patients, they are used by leading wound care facilities globally.
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight ‘s suite of commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
For more information, contact:
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
T. +1.647.362.4684
rsandler@moleculight.com
www.moleculight.com
Images:
Title: MolecuLight Successfully Completes SOC 2® Type l Audit and Accreditation
Download image: https://moleculight.box.com/s/9ycfyicr4fsxn8jfqdlo57q8sohqw1qz
SOURCE MolecuLight
Bacterial signaling across biofilm affected by surface structure, shows study
by Shelby Lawson, University of Illinois at Urbana-Champaign
3M Health Care introduces new 3M SoluPrep S Sterile Antiseptic Solution,
an optimized skin prep product for presurgical applications

Imitating the microenvironment of native biofilms using nanofibrous scaffolds to emulate chronic wound infections†
Jana Wächter, ORCID logo a Pia K Vestweber,a Nathalie Jung ORCID logo a and Maike Windbergs





The Potential of Topical Therapy for Diabetic Wounds: A Narrative Review
Umme Salma Rangwala • Fatema Tashrifwala • Nikita N. Egbert • Abuzar A. Asif


Diabetic Foot Osteomyelitis: Is it all the Same?
Gabriela Verónica Carro, MD, Fermín Martinez de Jesus, MD, and Anahí Ricci, MD


Wound Care Management: It’s More Than Just Dressing Change Or Bandaging
From biomaterial-based dressings to nanotherapeutics and bioengineered skin grafts, wound care management has evolved tremendously in the last couple of decades.

Lower Limb Arterial Ischemia: An Independent Risk Factor of Sudomotor Dysfunction in Type 2 Diabetes
Yuhuan Lv, Zheng Yang, Linyu Xiang, Meng Yu, Subei Zhao, Xiaoru Zhang, Rong Li


Nurses Make House Calls to Treat ‘Tranq’ Wounds for Users at Society’s Edge
Xylazine has permeated fentanyl supplies, causing wounds and amputations

ESPN star Michael Wilbon opens up about working through challenges of recent surgery
Wilbon was diagnosed with Type II diabetes after suffering a heart attack 15 years ago.


Impact of COVID-19 Crisis in the Management of Diabetic Foot Patients in
King Abdulaziz University Hospital, Jeddah, Saudi Arabia Rawan T. Harun • Abdullah A. Almohammadi • Maryam M. Alnashri • Sarah Alsamiri • Maram Alkhatieb

Diabetic Foot Ulcer Severity Exacerbated by the COVID-19 Pandemic
The COVID-19 pandemic may have negatively affected the timely diagnosis and treatment of diabetic foot ulcers.


Dermatologist Talks About the ABCs of Wounds and HS Support at Annual Meeting
Mar 17, 2023 Heidi Anne Duerr, MPH Hadar Lev-Tov, MD

Health workers carry wound care packs for ‘tranq’ users
Health workers carry wound care packs for ‘tranq’ users

Nonanimal Euglena gracilis-Derived Extracellular Vesicles Enhance Skin-Regenerative Wound Healing
Yuri Ko, Hwira Baek, Jee-Hyun Hwang, Youngseok Kim, Kyung-Min Lim, Junoh Kim, Jin Woong Kim




Fish Skin Grafts Versus Alternative Wound Dressings in Wound Care: A Systematic Review of the Literature
Mohamed Ibrahim • Haneen S. Ayyoubi • Layth A. Alkhairi • Hozaifa Tabbaa • Isaac Elkins • Ravish Narvel
Intermittent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance
Masaru Usui, Yutaka Yoshii, Stanislas Thiriet-Rupert, Jean-Marc Ghigo & Christophe Beloin

Study Finds Vomaris Bioelectric Technology Significantly Decreased Biofilm in Burn Patients
Sumit Puri, CIO, Max Healthcare Scott Arnold, Executive Vice President & CIO, Tampa General Hospital Georgia Mitchell, Director, Clinical and Medical Affairs, Stryker (Spine Division)
Antibacterial, Anti-Biofilm and Pro-Migratory Effects of Double Layered Hydrogels Packaged
with Lactoferrin-DsiRNA-Silver Nanoparticles for Chronic Wound Therapy Centre for Drug Delivery Technology, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur 50300, Malaysia

Weight-bearing physical activity in people with diabetes-related foot disease: A systematic review
Jaap Van Netten, Vera Fijen and and Sicco Bus.



Effect of ultrasound-supported wound debridement in subjects with diabetic foot ulcers: A meta-analysis
Haiting Chen, Ting Xiao, Ling Zhang, Ning Liu, Xia Liang, Tuodi Li, Jinying Wang, Yaozhong Peng, Yanping Liu, Jiali Xu

How to Make Dakin’s Solution
Many have wanted instructions for making the solution developed by the British Chemist Henry Dakin and the French Surgeon Alexis Carrel. To that end, we enclose the recipe.



REGENATIVE LABS ANNOUNCES PUBLICATION OF GROUNDBREAKING PAPER ON
WHARTON’S JELLY APPLICATIONS, OPENING THE DOOR FOR EXPANDED TREATMENT OF THE SI JOINT Dr. Albert Lai

Factors associated with severity and anatomical distribution of diabetic foot ulcer in Uganda:
a multicenter cross-sectional study Bienfait Mumbere Vahwere, Robinson Ssebuufu, Alice Namatovu, Patrick Kyamanywa, Ibrahim Ntulume, Isaac Mugwano, Theophilus Pius, Franck Katembo Sikakulya, Okedi Francis Xaviour, Yusuf Mulumba, Soria Jorge, Gidio Agaba & George William Nasinyama

Pain Management With Topical Ibuprofen in Partial-Thickness Burn Wounds and
Effects on Wound Healing: A Prospective Randomized Clinical Study Ali Emre Akgun, MD Merve Alkin, MD

Addressing the Variety and Complexity of Wounds through Innovations in Negative Pressure Wound Therapy
Amanda Estapa, ACNP-BC, CWS, FACCWS, DAPWCA Vice President of Clinical Services MedCentris Hammond, LA Dot M. Weir, RN, CWON, CWS Clinician Saratoga Hospital Wound Healing Gansevoort, New York Colin J. Traynor, DPM, CWSP Adjunct Assistant Professor Samuel Merritt University ORINDA, CA

Eliminating Bias in Clinical Trials: Endpoints, Interpretation, and Presentation with Dr. Suzanne Bakewell
Dr. Suzanne Bakewell, Chief Scientific Officer for skin care company Omeza

The Role of Personal-use Negative Pressure Wound Therapy with Enhanced Functionality
in Achieving Wound-related Treatment Goals: A Small Prospective Study Robert F. Mullins Joan Wilson Zaheed Hassan Bounthavy Homsombath Beretta Craft-Coffman Rey Paglinawan Inez Cregan Shawn Fagan

Wound Biomarkers: Measuring What We Manage
Windy Cole, DPM, CWSP

Unique Insights from Experts: Mechanisms for Managing Diverse and Challenging Wound Etiologies with Instillation Therapy
Elizabeth Faust, MSN, CRNP, CSWS, CWOCN-AP, DAPWCA CRNP, WOCN Tower Health System Leesport, PA Paul J Kim, DPM, MS, FACFAS, FAPWH Professor, Medical Director University of Texas Southwestern Dallas, TX

Diabetic Wound Care (video)
Wound Problem After Below Knee Amputation Treated with new age creams (boric acid and Hypericum perforatum ( Sarı kantaron ), Calendula officinalis , Neem oil ( Azadirachta indica ) and Centellin.


American College of Surgeons (ACS) Clinical Congress OCTOBER 22-26, 2023 (BOSTON, MA)
conferences and events

Circumferential Leg Ulcers With NPWT
Sandra Wainwright, MD

The Role of the Plastic Surgeon in Wound Care
Richard Simman Fuad-Tahsin Abbas Darren Gordon

Preliminary evaluation of dual-energy CT to quantitatively assess bone marrow edema
in patients with diabetic foot ulcers and suspected osteomyelitis

An Algorithmic Approach to Negative Pressure Wound Therapy: Home Healthcare and Beyond
FACULTY Todd Shaffett, DNP, FNP, CWS, FACCWS, DAPWCA President MedCentris Hammond, LA Dot Weir, RN, CWON, CWS Clinician Saratoga Hospital Wound Healing Gansevoort, NY

Primary Cutaneous Mucormycosis of the Lower Extremity in a Male Patient With Diabetes
Nicholas Chang James McKee Valerie Marmolejo Arnold Paul C. Cua

Recommendations for Wounds After Flaps and Grafts
Michael Desvigne, MD, FACS, CWS, FACCWS Dr. Desvigne will present practical examples and case studies regarding the treatment of flaps and grafts after procedures, such as metatarsal amputation and the treatment of non-healing wounds. Dr. Desvigne will also discuss tools and methods he has used in the treatment of these amputation sites and non-healing wounds.

Addressing Both the What and the Why of Clinical Documentation
by Caroline Fife, M.D.

Managing the Landscape of Wound Types Working from a Comprehensive Algorithm
Vickie R. Driver, DPM, MS, FACFAS System Wide Medical Director INOVA Wound Healing and Hyperbaric Centers Falls Church Virginia Barry University , Miami Shores Florida Anderson Island, WA Daniel L. Kapp, MD Plastic Surgeon Daniel Kapp MD Plastic Surgery and Wound Care West Palm Beach, Florida Andrew J. Rader, DPM, FAENS, FACFAOM, FASPS, FAPWCA, FACCWS…

Early Skin Temperature Characteristics of the Kennedy Lesion (Kennedy Terminal Ulcer)
Karen Lou Kennedy-Evans, RN, FNP, APRN-BC; Deanna Vargo, BSN, RN, CWS, FACCWS, CWOCN; Leslie Ritter, PhD, RN; Diane Adams, BSN, RN, CWCN; Suzanne Koerner, BSN, RN, CWOCN; Ellen Duell, APRN, CWOCN, ACNS-BC

Preventing Mortality in Patients with DFUs
BY LEONARD A. LEVY, DPM, MPH

Eugene artist uses ‘good, gross art’ to illustrate ailments worsened by homelessness
Anyone can get the wounds depicted, but a lack of access to hygiene can make them so much more likely. Tatiana Parafiniuk-Talesnick


From the labs. Biofilm buster
Nanocomposite coating inhibits biofilm formation during post-operative care



Fragile Feet and Trivial Trauma: Communicating the Etiology of Diabetic Foot Ulcers to Patients
Gustav Jarl, Jaap van Netten, and Peter Lazzarini with another important contribution

Uncommon Wounds: Frostbite and Winter Woes
By Lauren Lazarevski, RN, BSN, CWOCN

Wound infections: an overview
Martha Williams
Responses to “Who Should Assess and Stage Pressure Injuries in Hospitalized Patients?”
Michael A. Bain, Garrett Wirth, Robert X Murphy
Wound swab quality grading is dependent on Gram smear screening approach
Shawn T. Clark, Jessica D. Forbes & Larissa M. Matukas

Design, development, in-vitro and in-vivo evaluation of polylactic acid-based multifunctional
nanofibrous patches for efficient healing of diabetic wounds Isra H. Ali, Islam A. Khalil Ibrahim M. El-Sherbiny







Risk factors for wound healing complications after revascularization for MMD with complete Y-shaped incision
Chenchao Wang, Hongwei Li, Yang Dong, Hao Wang, Dongpeng Li, Chengbin Zhao, Lei Cao, Kaiwen Sun, Jiefeng Geng & Bo Yang

Diabetic Foot: Facts and Figures from DF Blog
David Armstrong
Congress Takes a Positive Swing at an Unsung Public Health Crisis: Preventing Pressure Injuries in Veterans Affairs Facilities
Padula, William V. PhD; Black, Joyce M. PhD, RN; Garcia, Aimee MD; Mishra, Manish K. MD, MPH
REGENATIVE LABS AND BROTHERS IN ARMS FOUNDATION ANNOUNCE NEW
PARTNERSHIP TO OFFER STATE OF THE ART CARE TO INJURED VETERANS WITH 5 MILLION DOLLAR DONATION
Both organizations call on physicians to assist Veterans.
PENSACOLA, Fla. , Feb. 27, 2023 /PRNewswire/ — Regenative Labs (Regenative), a leading HCT/P manufacturer, is partnering with Brothers in Arms Foundation (BIAF), a 501©3 nonprofit providing logistical and financial support to wounded, ill, injured and fallen Marines, sailors, and the families of those who served. Together, both organizations are dedicated to giving back and improving the quality of life for those who have served our country.
Regenative will donate approximately $5 million dollars of its state-of-the-art Wharton’s Jelly product, ProText™, to BIAF which will facilitate the care of qualifying veterans. To bring this care to veterans, Regenative and BIAF need the help of physicians to apply the product.
Physicians across the country may join in supporting our veterans by making a qualified donation of their time under the Brothers in Arms Foundation 501©3 non-profit.
“We’re extremely proud of this partnership,” shared Regenative Labs CEO, Tyler Barrett. “Ultimately our products are about improving patient quality of life. My grandfather served in the Marines in Korea, and so being able to serve those like him in unison with the mission of Brothers in Arms is important to me,” Barrett concluded.
Regenative’s Wharton’s Jelly product is a structural connective tissue allograft intended for homologous use to replace or supplement missing or damaged tissue directly at the site of a structural defect. Some doctors have used the product for muscle and cartilage tears and to replace missing or damaged tissue due to wounds and tissue defects, instead of masking the underlying connective tissue issue.
Regenative has been tracking patient outcomes of applications of their product for over two years. Retrospectively, the results have shown improvements greater than 33 percent in patient reported WOMAC improvement.
It is Regenative’s and BIAF’s goal to provide veterans with the best quality of life possible.
“We’re committed to bettering the quality of life for our veterans,” said Brothers in Arms Foundation President, Phillip Noblin. “This new, innovative treatment will give them the hope and support needed to heal. The partnership with Regenative Labs will further our mission of serving those who’ve served us,” said Noblin.
Physicians interested in taking care of qualified veterans by donating their services through a 501©3 non-profit may visit: https://regenativelabs.com/biaf-physician-time-and-services-donation/
Veterans who wish to learn more, or to refer their physician today may visit: https://regenativelabs.com/biaf/
One can make a monetary donation through the BIAF.
About Regenative Labs: Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with daily challenges of innovations in healthcare, the company provides non-addictive, non-invasive options for patients. Regenative Labs’ expert product research and development team complies with FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines. Learn more at Regenative’s website: www.regenativelabs.com
About Brothers in Arms Foundation: Brothers in Arms Foundation is a 501c3 non-profit founded in 2009 that exists to provide financial and logistical support to wounded, ill, injured and fallen Marines, sailors, and the families of those who served within the Marines Special Operations community. Over the years, the foundation has supported active-duty Marines, sailors, veterans and military families with programs and services ranging from financial assistance, funding vocational and quality of life initiatives, as well as funeral support and childcare. Learn more at:
https://www.brothersinarmsfoundation.org
SOURCE Regenative Labs
Foot ulcers in systemic sclerosis escape surgical amputation
Dr Giuseppina Abignano

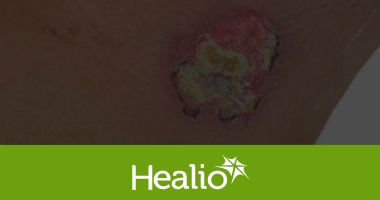
Albuminuric diabetic kidney disease increases risk for foot ulcers
Vincent Rigalleau

New paper targets prevention of foot and lower limb diseases
Dr. Peter Roberts


Chronic Venous Insufficiency and Lymphedema With Papillomatosis Cutis Lymphostatica,
Hyperkeratosis, and Skin Ulcers: A Case Report Jayani Senanayake • Sanket Chaudhari • Rangin Haji Rahman • Sally Madanat • Frederick Tiesenga


Roundtable Discussion from The American College of Clinical Wound Specialists (ACCWS)
Frank Aviles, PT, CWS, FACCWS, CLT-LANA, ALM, AWCC, DAPWCA


Marching Forward With Global Pressure Injury Data
Elizabeth A. Ayello, R. Gary Sibbald



Bilayer hydrogel dressing with lysozyme-enhanced photothermal therapy for biofilm eradication
and accelerated chronic wound repair biofilm eradication and accelerated chronic wound repair Yizhen Wang, Qijun Lv, You Chen, Langtao Xu, Miao Feng b, Zhiyong Xiong, Jiajun Li, Jie Ren, Jie Liu, Bo Liu
Long-term Follow-up in Restoring Soft Tissue Deficits with A Novel Human Adipose Allograft Matrix*
Speaker: Matthew Regulski, DPM FFPM RCPS (Glasgow)

Wound Masterclass | Emerging Technologies in Clinical Practice
Yuanwen Jiang, Artem Trotsyuk, Simiao Niu, Dominic Henn, Kellen Chen, Chien-Chung Shih, Madelyn R. Larson, Alana M. Mermin-Bunnell, Smiti Mittal, Jian-Cheng Lai, Aref Saberi, Ethan Beard, Serena Jing, Donglai Zhong, Sydney R. Steele, Kefan Sun, Tanish Jain, Eric Zhao, Christopher R. Neimeth, Willian G. Viana, Jing Tang, Dharshan Sivaraj, Jagannath Padmanabhan, Melanie Rodrigues, David P.…

First patients enrolled in trial of RLF-TD011 wound spray for EB
by Patricia Inácio, PhD | February 17, 2023

Cicatrization of Oncological Wounds by Autologous Fibrin Biopolymer Dressing Treatment: A Case Series
Chayane K.L. de Carvalho Beatriz Luci Fernandes Michel M. Dalmedico Mauren Abreu de Souza

VASCULAR Scientific Sessions
Presented by the Society for Vascular Medicine September 7 — September 10, 2023 Grand Hyatt • Washington, DC conferences and events

The Amputee Coalition 2023 National Conference
Renaissance Orlando at SeaWorld®, August 2 -5, 2023 conferences and events

Skin Substitute Reimbursement and Coding Best Practices Webinar Series
Dr. Jeffrey Lehrman, DPM, FASPS, MAPWCA, CPC, CPMA Terrence Mabry – MIMEDX Regional Reimbursement Director

ADVANCED WOUND CARE SUMMIT | 12-13 JULY, 2023
BOSTON, USA conferences and events

HOSPITALS LOOK TO IMPROVE WOUND CARE WITH SOFTWARE
BY ERIC WICKLUND | OCTOBER 07, 2022

Wild on Wounds – September 13-16 Hollywood Florida
conferences and events

Worldwide Research Trends on Diabetic Foot Ulcers
(2004-2020): Suggestions for Researchers Pin Deng, Hongshuo Shi, Xuyue Pan, Huan Liang, Shulong Wang, Junde Wu, Wei Zhang, Fasen Huang, Xiaojie Sun, Hanjie Zhu, Zhaojun Chen


Speaker offers pearls in epidermolysis bullosa therapies
Rebecca L. Forand

Severe wound infection by MRCNS following bilateral inguinal herniorrhaphy
Yao Du, Song Han, Yue Zhou, Hai Feng Chen, Yao Liang Lu, Zhi Yuan Kong & Wei Ping Li


Lower-extremity pressure, staging, and grading thresholds to identify chronic limb-threatening ischemia
Justin Chin-Bong Choi, Jorge Miranda, Neal R Barshes