Guilherme de Castro-Santos, Patric Emerson Oliveira Gonçalves, and Túlio Pinho Navarro


Guilherme de Castro-Santos, Patric Emerson Oliveira Gonçalves, and Túlio Pinho Navarro

Michelle Guo, Mary M McDermott, Philip Greenland

lessons from the Medicare Database (2015–2018) | Published Online:14 Jul 2021

February 22-25 San Antonio TX conferences and events

Renata Bienias Marco Feulner Stefan Hergenröder Hannes Schrammel Lisa Spreitzer Elzbieta Szkile Adam Zielinski Claas Roes

Resorbable Glass Fiber Matrix in the Treatment of Diabetic Foot Ulcers David G. Armstrong, DPM, MD, PhD

David Navazio 2/7/2023

28th – 30th of September, 2023 – Anaheim, California conferences and events

Paul Glat Lisa Gould Lael J Pickett Douglas M Arm Clinical Trial Clinical Research Wound Healing Diabetic Foot Ulcer Clinical Trial Bias Minimizing Bias
Findings from a New Clinical Study Using MolecuLight Imaging of Diabetic Foot Ulcers Prompts New Diagnostic Terminology Enabling Proactive Infection Management
TORONTO, Feb. 14, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated bacterial loads in and around wounds, announced the publication of “Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers“1 in International Wound Journal. The publication reports on the analysis of 138 diabetic foot ulcer wounds, highlighting the frequent presence of healing delays and elevated bacterial burden as identified through standard clinical assessment, fluorescence imaging (MolecuLight i:X®), and quantitative microbiology.
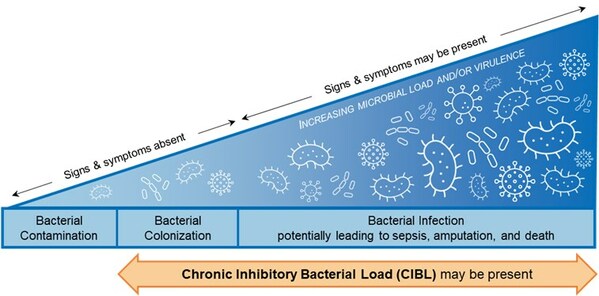
Chronic inhibitory bacterial load (“CIBL”) on the bacterial-infection continuum. Based on the International Wound Infection Institute (IWII) 2022 wound infection continuum (CNW Group/MolecuLight)
The emergence of data on the link between bacterial load and healing over the last decade, together with this current study, prompted study authors David G. Armstrong, Michael E. Edmonds, and Thomas E. Serena to define new clinical terminology, chronic inhibitory bacterial load (CIBL). CIBL is defined as “the chronic presence of bacterial microorganisms in a wound or its surrounding tissue at loads which can damage tissues and be inhibitory to healing, as well as require clinical intervention, with or without the presence of clinical symptoms”.
MolecuLight fluorescence imaging is currently the only way to detect and locate CIBL at the point of care. This term enables the proactive diagnosis of CIBL early along the bacterial-infection continuum, to facilitate its targeted removal, promote healing, and prevent the sequelae of infection in frequently asymptomatic diabetic ulcers.
Key findings of the study include:
Infection prevention is a key goal of CIBL’s introduction, adoption, and management. CIBL is the result of these seasoned wound care clinicians’ long-time advocacy for proactive wound management as they see firsthand the devastating consequences of delayed treatment. “Infection is the greatest destroyer of the diabetic foot. It is the final common pathway for most amputations, and we need to fight it as early as possible in its natural history”, says Dr. Michael E. Edmonds, one of the paper’s authors and Consultant of Diabetologist at the Diabetic Foot Clinic, King’s College Hospital Foundation Trust in London, UK. “CIBL localization and proactive management is a crucial strategy in reducing unnecessary amputations and saving lives”, he concludes.
As MolecuLight is the only device capable of detecting elevated bacterial loads in wounds in real-time, regions of CIBL can be non-invasively and accurately detected and mapped. The device provides clinicians with immediate feedback to guide their therapeutic decision-making process in a number of clinical settings from the outpatient clinic to the operating room. Multiple routine procedures are enhanced by its proven capabilities, such as debridement, wound hygiene, and preparation for advanced therapies resulting not only in better outcomes,3,4 but more rational resource consumption and antimicrobial stewardship.4
“There is also a meaningful role for fluorescence imaging with MolecuLight in antimicrobial stewardship. This is critical considering that approximately 70% of patients with diabetic foot ulcers are prescribed antibiotics at some point during their care, and over 80% are prescribed antimicrobial dressings3, often in a haphazard manner”, says Dr. Thomas Serena, study author and the Founder and Medical Director of The SerenaGroup®. “Diagnostic uncertainty has been listed as a key factor in antibiotic overuse in wound care. Fluorescence signals as a real-time imaging biomarker of CIBL could enable clinicians to more effectively leverage hygiene-based strategies to remove bacteria rather than resorting to antibiotics”.
“The definition of an infection’s genesis and its resolution is a clinical one”, notes Dr. David G. Armstrong, study author, Professor of Surgery at the University of Southern California, and founder and co-Director of the Southwestern Academic Limb Salvage Alliance (SALSA). “The problem is that many objective local signs may be blunted in the chronic wound and it is likely that we are not yet effectively measuring what we manage. Fluorescence imaging of chronic inhibitory bacterial load (CIBL) is positioned to potentially change contemporary paradigms of wound management. We are hopeful that this new clinical term, CIBL, can be a key indicator to enable pre-infection intervention such as debridement or modification of wound therapy.”
The MolecuLight i:X and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 65 peer-reviewed publications involving 1,500 patients, they are used by leading wound care facilities globally.
References
1 Armstrong DG, Edmonds ME, Serena TE. Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers. Int Wound J. 2023;20(2):554-566
2 Wounds International (2022) International Consensus Update 2022 International Wound Infection Institute (IWII) Wound Infection in Clinical Practice: Principles of best practice. Available from https://woundinfection-institute.com/
3 Price N. Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: retrospective analysis of 229 foot ulcers. Diagnostics (Basel). 2020;10(11):927.
4 Rahma S, Woods J, Nixon JE, Brown S, Russell DA. The use of point-of-care bacterial autofluorescence imaging in the Management of Diabetic Foot Ulcers: a pilot randomised controlled trial. Diabetes Care. 2022;45:1601-1609.
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight ‘s suite of commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
SOURCE MolecuLight

Where: San Diego Convention Center, San Diego, California, USA When: Friday, June 23, 2023 (beginning at 10:30 a.m.) through Monday, June 26, 2023 (concluding at 4:15 p.m.) conferences and events

DATE July 13 – 16PLACE Nashville, TN conferences and events



Donor Site Wounds in Cases of Split-thickness Skin Graft Iyer Sandhya P Prabhakar Subramaniyan Gade Sujata V

September 28-30, 2023

Samuel Adegboyega, DPM

3-5 May 2023 in Milan, Italy Sebastian Probst, Luc Teot, Laura Stefanon conferences and events


Jeanine Maguire, Vice President, Skin Health & Wound Care Integrations, Genesis


Laura Kaiser, RN, MSN, APRN-BC, CNS, WOCN

Raleigh, NC, March 11–12, 2023

the Kingsgate Conference Centre, 26 – 27 April conferences and events


Harry Schneider, DPM, FACFAS

Marriott Marquis San Diego Marina 333 W Harbor Drive San Diego, California 92101 conferences and events

Based on the latest evidence and innovations in wound care, WoundCon Spring 2023 offers practical strategies that you can immediately implement into your practice. Featuring free registration for licensed healthcare professionals and the convenience of interactive education with world-renowned specialists on March 10, this a must-attend CME/CNE meeting. conferences and events

Future Advancements and Strategies in Wound Healing and Critical Care conferences and events

IEA Professional Development Center 3440 Liberty Drive Springfield, IL 62704 conferences and events

MARSHALL ARENA MILTON KEYNES conferences and events

In-Person Event • Apr 26 – 29, 2023 • National Harbor, Maryland, USA conferences and events
The Edges of Wound Care 2023 Karen L. Andrews, M.D. Jennifer L. Blanco, M.S.N., R.N., CNOR Brianna M. Skrukrud, APRN, C.N.P. Danielle T. Vlazny, P.A.-C., M.S. Saranya P. Wyles, M.D., Ph.D. conferences and events

conferences and events

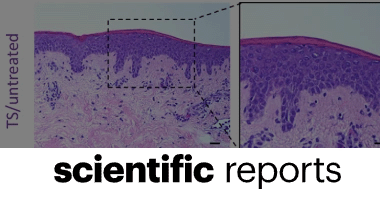
Christopher Doherty, Charlotte V. Byrne, Sajwa Baqader, Cecile El-Chami, Andrew J. McBain & Helen A. Thomason

Wed, Feb 8, 2023 2:00 PM – 3:00 PM EST

Anne Lin, PharmD, BCPS

Earl Blumenauer
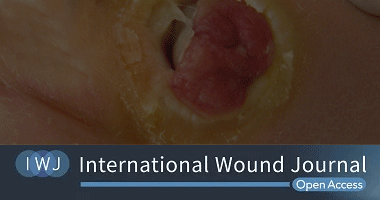
David G. Armstrong, Michael E. Edmonds, Thomas E. Serena

24TH FEBRUARY 2023 AMERICA SQUARE CONFERENCE CENTRE conferences and events


LOS ANGELES FEBRUARY 9-12 conferences and events


NASHVILLE JULY 13-16, 2023 conferences and events

San Diego, California • March 17-18, 2023 conferences and events

Elizabeth Ansert, MA, DPM, MBA

By Michael Greenwood, M.Sc. Reviewed by Emily Henderson, B.Sc.

Neil Skolnik, M.D and John J. Russell, M.D

conferences and events

10 – 13 May 2023 | World Forum The Hague – The Netherlands conferences and events



by Marisa Wexler, MS

Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact Beata Mrozikiewicz-Rakowska, Ilona Szabłowska-Gadomska, Dominik Cysewski, Stefan Rudziński, Rafał Płoski, Piotr Gasperowicz, Magdalena Konarzewska, Jakub Zieliński, Mateusz Mieczkowski, Damian Sieńko, Tomasz Grzela0, Maria Noszczyk1, Barbara Paleska, Leszek Czupryniak, Malgorzata Lewandowska-Szumiel

to the Problem of Diagnostics, Statistics and Standardisation Pavel Lukin, Alex G Kuchumov, Mikhail F Zarivchatskiy, Tatyana Kravtsova


from 2019 BY MARC A. BRENNER, DPM, STANLEY R. KALISH, DPM, PRADEEP ALBERT, MD, AND ALBERT RAMINFARD, DO


Carrie Nagorka, Senior Editor
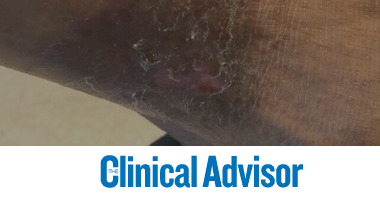
Valerie K. Sabol, PhD, MBA, ACNP, GNP, ANEF, FAANP, FAAN
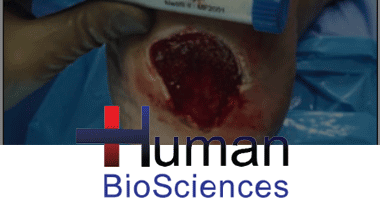
BY MARC A. BRENNER, DPM, STANLEY R. KALISH, DPM, PRADEEP ALBERT, MD, AND ALBERT RAMINFARD, DO
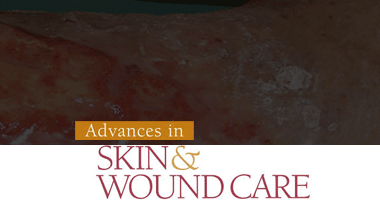
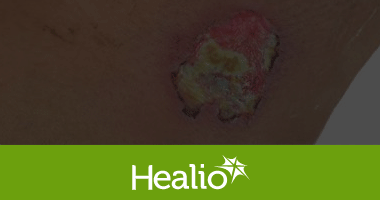
Karl G. Stonecipher, MD
An updated systematic review and meta‐analysis
Shinya Sugimoto & Yuki Kinjo
NOVEL APPLICATION OF UMBILICAL CORD FLOWABLE TISSUE ALLOGRAFTS IN DECUBITUS ULCERS
PENSACOLA, Fla., Jan. 23, 2023 /PRNewswire/ — A case study analysis of two patients has been presented by Regenative Labs (Regenative), a leading HCT/P manufacturer, in collaboration with Dr. Michael Lavor of Saguaro Surgical. This case study demonstrates the use of Wharton’s Jelly, a connective tissue, allografts in combination with standard of care wound practices to accelerate the healing of refractory, Stage IV sacral wounds in paralyzed patients.
In reference to what he’s seen in the patient population regarding Wharton’s Jelly allografts, Lavor shared, “the patients have begged for more because it is the only thing that has helped them. I believe this is an excellent step prior to surgery, and will save hundreds of thousands of dollars.”
This case study demonstrates an application of Wharton’s Jelly allografts in late-stage sacral decubitus ulcers (SDU), also known as pressure sores, with associated tunneling in combination with standard of care. In the future, research may focus on the frequency and combination of procedural techniques that most efficiently promote granulation tissue formation and volumetric contracture of deep wounds with Wharton’s Jelly allografts.
“This, for wounds, is excellent for closing tunnels,” explained Dr. Lavor.
It was reported that for the first time in ten years, one patient experienced a highly accelerated wound closure rate, and observed volumetric reduction in the wound bed, healthy granulation tissue, and the resolution of deep tunneling. One patient achieved full one closure and epithelization.
Both patients in the presented case study had SDU classified as Stage IV with tissue loss and bone or tendon involvement. One patient had previously experienced a mid-sacral pressure sore with exposed tendon, bone, and tunneling for ten years. The other, had an ischial pressure sore with the same features that persisted for 30 months. After failing multiple conservative treatments such as wound vac placement, antibiotics, wet-to-dry dressings, and silver sulfadiazine dressings, both patients received several applications of Regenative’s Wharton’s Jelly allograft.
In both cases, after eight months of standardized wound care treatment combined with six applications of Regenative’s Wharton’s Jelly allograft, the wounds contracted by over 90% in depth, tunneling, and diameter.
Annually, thousands of individuals are affected by SDU. Treatment for these conditions is costly and far from perfect, with prices as high as an eye-watering 240,000 dollars for skin flap surgery. Inevitably, Stage II pressure ulcers can become serious if not handled swiftly. When deep, tunneling, and with both tendon and bone involvement, such as the two patients in this case study, late-stage pressure sores occur, and pose a great challenge to medical professionals.
Regenative is committed to providing patients with alternative options, and through what may be revealed in these studies, offering proven treatments to better address the root cause of their pain.
Regenative hopes to enlist physicians to take part in studies regarding uncovered uses. Physicians will have their outcomes highlighted, furthering the understanding of regenerative medicine and uncovering new applications for this groundbreaking field of medicine.
“The research at Regenative is very promising, and we’re calling on physicians across the country to engage with us and advance regenerative medicine,” shared Regenative Labs CEO, Tyler Barrett.
Contact Regenative to get your practice involved today.
About Regenative Labs: Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with the daily challenges of innovations in healthcare, the company provides non-addictive, non-invasive options for patients. Regenative Labs’ expert product research and development team comply with FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines. Learn more at Regenative’s website: www.regenativelabs.com
About Dr. Michael Lavor: Dr Michael Lavor has worked for over 28 years bringing the highest quality of medicine to his patients. He is currently based out of Arizona, and is planning to open his own practice within the first 2 quarters of 2023. In addition to his work as Assistant Medical Director at Saguaro Surgical, Dr. Lavor was a member of the Trauma Team at Tucson Medical Center where he also served as Chairman of the Department of General and Vascular Surgery. Dr. Lavor was board certified in General Surgery and was a fellow in the American College of Surgeons, past president of the Rocky Mountain Vascular Surgical Society, a Fellow in the Southwestern Surgical Congress, a member of the Tucson Surgical Society, a member of the International Society of Endovascular Surgery and the Pima County Medical Society. Lavor served in the Navy for ten years as a Navy Corpsman with the Marines; he returned to service in 2009 and served as a Commander / OIC of a wound surgical base in Afghanistan from 2012-2013. He also was a Clinical Associate Professor at the University of Arizona Medical Center Department of Surgery.
This article was originally published here
Suwon, Korea, Min Ji Kim, Yon Soo Jeong, Hee Joung Kim, Hyung Min Hahn, Duy Quang Thai, Jae Lee

By Nancy Morgan RN, BSN, MBA, WOC

Indication #3: Radiation Injuries Denise Nemeth, MPAS, CWS Jayesh B. Shah, MD, MSc, UHM ABPM, CWSP, FAPWCA, FCCWS, FACHM FUHM, FACP

More than 500 abstracts featuring late-breaking wound care research, new advances and techniques to improve care and outcomes for patients were submitted for poster at the 2023 event, co-located with the Diabetic Limb Salvage meeting.
HMP Global, the leading omnichannel healthcare events and education company, today announced that its 2023 Symposium on Advanced Wound Care (SAWC) Spring and Wound Healing Society (WHS) received a record-breaking number of abstract submissions for the event taking place April 26-30, strengthened by a new partnership co-locating the symposium with the Diabetic Limb Salvage conference.
Now in its 36th year, SAWC Spring | WHS is the leading meeting dedicated to the research, management, treatment, and prevention of wounds; and through the partnership with DLS, this year’s event will feature more limb salvage-focused topics on the conference agenda. The meeting is the premier multidisciplinary forum to connect practitioners, researchers, and students with the foremost experts in wound care to improve patient outcomes through education.
Symposium participants will have access to 450 posters featuring late-breaking wound care research, new advances, strategies, and techniques to improve care and outcomes for patients. More than 500 abstracts — a record number — were submitted to undergo the peer review process for poster consideration.
In addition to presenting posters in person at SAWC Spring | WHS | DLS, wound care researchers can elevate their work further by submitting abstracts for publication in the field’s preeminent, peer-reviewed journal WOUNDS, focusing on the latest advances in wound care and wound research. WOUNDS is indexed in MEDLINE/PubMED and publishes research and commentary on tissue repair and regeneration, biology and biochemistry of wound healing, and clinical management of various wound etiologies. Submission information and guidelines are available on HMP Global’s Wound Care Learning Network.
“The record-breaking number of abstract submissions this year is a testament to the dedication of the wound care community to advancing their knowledge and skills,” said Tiffney Oliver, Vice President, Wound Care Learning Network, HMP Education. “For our 2023 meeting, we are excited to offer a world-class lineup of educational sessions as well as a record number of abstracts about the latest research in wound care.”
Submitted abstracts are blind reviewed by a panel of expert judges, based on specific criteria for the category in which it was submitted. Researchers may also be considered for poster grand rounds, oral abstracts, SAWC Young Investigator, and highest scoring abstract honors.
“We are excited to host one symposium for every member of the wound care team, allowing us to provide the highest caliber training and education that all clinicians can incorporate into their practice,” said WHS President Dr. Kenneth Liechty, Division Chief of Pediatric Surgery and Director of Fetal Medicine, University of Arizona, and Surgeon in Chief of Diamond Children’s Hospital. “The quantity and high caliber of the posters presented this year spotlights the most up-to-date research on wound care and limb salvage. This level of exposure to innovation is unparalleled in the wound care community.”
Posters will be on view from 7:30 a.m. to 5 p.m. Friday, April 28, and from 7:30 a.m. to 5 p.m. Saturday, April 29. In addition, SAWC Spring | WHS | DLS participants will have the opportunity to interact with the researchers during the Poster Reception and Awards Presentation from 7:15-8:30 p.m. on April 28, presented by WOUNDS.
Educational Program
The SAWC Spring | WHS | DLS agenda features more than 80 high-impact sessions from expert presenters led by the giants and emerging voices in the field, providing more than 25 CME/CE credits. Participants will have access to sessions in traditional as well as new formats, including hands-on workshops, rapid-fire, case-based, and patient panels. Learning tracks encompass the business of wound care as well as separate tracks through DLS and WHS.
“We have partnered with the Wound Healing Society for 15 years, providing a robust educational experience for meeting participants, and this year will be even stronger with the addition of multiple topics on amputation prevention, said SAWC Spring Co-Chair Dr. Robert S. Kirsner, Harvey Blank Professor and chairman, Dr. Philip Frost Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine. “No other wound care conference offers this level of education, advanced state-of-the-art clinical reviews, and emerging research findings.”
The interdisciplinary agenda is designed for every aspect of wound research, prevention, and healing, with an important focus on limb salvage. Sessions are designed for all members of the wound care team, including physicians, nursing professionals, podiatrists, physician assistants, physical therapists, researchers, scientists, dietitians, and healthcare, sales, and marketing professionals.
For more information or to register, visit sawcspring.com.
ABOUT HMP GLOBAL
HMP Global is the force behind Healthcare Made Practical — and is an omnichannel leader in healthcare content, events, and education, with a mission to improve patient care. The company produces accredited medical education events — in person and online via its proprietary VRTX virtual platform — and clinically relevant, evidence-based content for the global healthcare community across a range of therapeutic areas. Its brands include the HMP Global Learning Network, healthcare’s most comprehensive source for news and information; Psych Congress, the largest independent mental health meeting in the U.S.; the Evolution of Psychotherapy, the world’s largest independent educational event for mental health professionals; the Leipzig Interventional Course (LINC), the leading, global gathering for interdisciplinary cardiovascular specialists; EMS World Expo, North America’s largest EMT and paramedic event; and the Symposium on Advanced Wound Care (SAWC), the largest wound care meeting in the world. For more information, visit hmpglobal.com.
This article was originally published here

Hoang Thanh Tuan Vu Quang Vinh Tran Van Anh Tong Thanh Hai Tran Quang Phu Trinh Tuan Dung


and Lower Urinary Tract Infection Abdur Rehman • Hassan Raza • Beenish Fatima Zia • Fatima Farahi • Meeran Asher Syed

Current Insights, Regional Developments, Demand and Forecast to 2030 By Coherent Market Insights Published January 20

Jarrod Shapiro, DPM PRESENT Practice Perfect Editor

Christopher Barrett, DPM, CWS, MAPWCA

Therapy to the Feet and Hands: A Case Report Austin Rollins Kristie Ho Luis G. Fernandez Marisse A. Lardizabal Bryan Roth Sean F. O’Keefe Marc R. Matthews

Original research articles, review articles and technical communications are welcome. Initial submissions are due by June 1, 2023. Edited By: Boris Hinz, PhD, Robert Kirsner, MD, PhD, Heather Powell, PhD

By Rachael Zimlich, RN, BSN
Integrating Traditional Indigenous and Western Health Models for Improved Outcomes

A Case Report Karina Karina Dinar Rahmania Hubert Andrew Krista Ekaputri Johannes Albert Biben Nungki Ratna Martina

January 24th – Register

DeepView® accuracy increases to 86% in diagnosis of DFU healing potential on day one

Vilayvanh Saysoukha, DPM, DABPM, FACPM, FASPS, AACFAS

ISET speakers review the gains to be made by mastering the essentials, with the ultimate goal of avoiding amputation. by Caitlin E. Cox


for treating diabetic foot infections at Wounds UK – 3 November 2022

Shuli Chou, Huating Guo, Franz G. Zingl, +8, and Xiangyu Mou


Zhisheng Chasen Shao, BA Khurram Khan, DPM

4-6 September 2023 QEII Centre, London, UK

Mark Hinkes DPM FACFAS FAPWCA DABFAS

Dr. Charlotte Schwenner



Johnson & Johnson Services, Inc., 3M, Baxter

Shannon Kody, MD Alex G. Ortega-Loayza, MD, MCR

Jessy Nellipudi, Caleb Stone

Negin Shamsian, Chien-Chung Shih, Madelyn R. Larson, Alana M. Mermin-Bunnell, Smiti Mitta, Jian-Cheng Lail, Aref Saberil, Ethan Beard, Serena Jing, Donglai Zhong, Sydney R. Steele, Kefan Sunl, Tanish Jain, Eric Zhaol, Christopher R. Neimeth, Willian G. Viana, Jing Tang, Dharshan Sivaraj, Jagannath Padmanabhan, Melanie Rodrigues, David P. Perrault, Arhana Chattopadhyay, Zeshaan N. Maan, Melissa C.…

A Few Weeks Becomes Reality With Circularity’s Groundbreaking Biotech, D’OXYVA Via Upcoming IPO By Rhodri Collins


By Nancy Morgan RN, BSN, MBA, WOC

F Mohamad, Raghad R Alzahrani, Ahlam Alsaadi, Bahauddeen M Alrfaei, Alaa Eldeen B Yassin, Manal M Alkhulaifi, Majed Halwani


Salomão Fernandes, Rita Sérvio, Ana Rita Silva, Raquel Tavares, Paulo Rodrigues

Submitted by Janet Wolfson on January 7th, 2023




JELLY ALLOGRAFTS IN POST-SURGICAL BREAST REDUCTION CARE


Submitted by Alton R. Johnson Jr. on January 6th, 2023




Madeline A Bone, Sharon Latimer, Rachel M Walker and Brigid M Gillespie


Jennifer Spector, DPM, FACFAS, Assistant Editorial Director
By Margherita Cole on December 27, 2022

Found to Be Safe, Efficacious By Tim Smith

Amanda Westfall, DPM, FACFAS

A Breakthrough in the Management of Difficult to Heal Diabetic Foot Ulcer – an Innovative Film – Forming Wound Dressing StrataGRT/Stratamed from Stratpharma Switzerland Dr. Shanna Fraser and Dr. Jaminelli Banks

Therapy Drapes Amy K. McNulty, Robert Wilkes, Jason Bjork, Michael Turnbull, James Sieracki

care: three descriptive cohorts Keryln Carville, Janine Alan and Joanna Smith


Therapy on Chronic Venous Ulcer Healing Sheetal Uttaray, Venkata Vineeth Vaddavalli, Ajay Savlania, Arunanshu Behera, Lileswar Kaman, Ujjwal Gorsi

Agonist Medications for Diabetes Robert G. Smith, DPM, MSc, RPh, FNAP

Submitted by Christine Miller

Antimicrobial Ointment on a Chronic Wound Microbiome: A Secondary Analysis Associating Clinical and Laboratory Findings Matthew F. Myntti Patricia Stevenson Dianne Porral Valerie Y. Hayes

A Historical Perspective Christine Miller, DPM, PhD

March 4 & 5, 2023, to be held in ADNEC, Abu Dhabi, United Arab Emirates.

Pressure Injury Prevention: A Descriptive Cross-Sectional Study Didem Kandemir, PhD Yasemin Ozhanli, PhD, RN Hatice Erdogan, PhD, RN Zeynep Temiz, PhD


Notes on The Wounds Australia biennial national conference


in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives Authors Qin W, Wu Y, Liu J, Yuan X, Gao J

organisation of excisional wounds in a mouse model Hendrik Lintel BS, Darren B. Abbas MD, Duncan J. Mackay MD, MBA, Michelle Griffin MBChB, PhD, Christopher V. Lavin MD, Charlotte E. Berry BS, Nicholas J. Guardino BS, Jason L. Guo PhD, Arash Momeni MD, Donald R. Mackay MD, DDS, Michael T. Longaker MD, MBA, Derrick C.…

by Marie-Morgane Le Moel with Daniel Lawler in Paris

and their potential pathophysiologic factors: a systematic review and meta-analysis


Wound Healing Complications

Emission Property for Efficient Photodynamic Killing of Bacteria and Wound Healing Authors Hou B, Yang F, Hu C, Liu C, Xiao X, Chen Y, Huang X, Xie S

Kathleen D. Schaum, MS

the moderate to severe diabetic foot infection: a meta-analysis Xinyi Lin, Yan Wu, Hong Huang, Ruihan Peng, FuHua Huang, Lvrong Hong, WenZhuan Chen

With Facilitated Transition Discharge Program for Patients Receiving Negative Pressure Wound Therapy Leila Boti Laura Soloway Deb Myers Dinu Pillai Javad Zabihi
invading human diabetic wounds and accelerates wound closure in diabetic mice

By Nancy Morgan, RN, BSN, MBA, WOC, WCC, DWC, OMS


Steven Bowers

Bronwyn L. Dearman BSc(Hons), PhD, Steven T. Boyce PhD, John E. Greenwood BSc(Hons), MBChB, MD, DHlthSc, FRCS(Eng), FRCS(Plast), FRACS

James V. Stillerman, MD, CWSP

Attending at Arba Minch General Hospital, Southern Ethiopia Authors Alelign D , Tena T, Tessema M, Kidanewold A
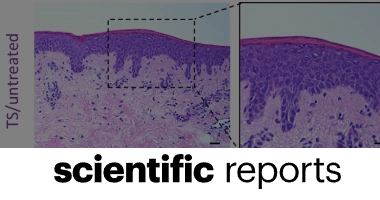
Camila Cárdenas-Calderón, Valentina Veloso-Giménez, Tamara González, Aniela Wozniak, Patricia García, Sebastián San Martín, Juan F. Varas, Ivo Carrasco-Wong, Mario Vera & José Tomás Egaña

Our free advanced study days promote the best practices in skin health and wound healing Sarah Gardner, Jo Dumville, Naseer Ahmed, Leanne Atkin, Rebecca Elwell, Kate Williams

Yasser A. Elghoneimy, Ali A. Alkabah, Hasheem M. Alalsayedsalih, Ali J. Almanyan, Hassan A. Alibrahim, Mostafa H. Albokamsin, Shadi A. Alshammary, Fahd A. Makhdom
in their 2023 Standards of Care in Diabetes
OCEANSIDE, Calif., Dec. 13, 2022 /PRNewswire/ — Advanced Oxygen Therapy Inc. (AOTI), the global leader in noninvasive topical oxygen wound healing solutions, announced today that the American Diabetes Association has awarded an “A” grade recommendation for utilizing adjunctive topical oxygen therapy in treating Diabetic Foot Ulcers (DFU) in their 2023 standards of care in diabetes, the preeminent Clinical Practice Guidance (CPG) in the space, which was published today online: https://diabetesjournals.org/care/issue/46/Supplement_1

ADA – Amputation Prevention Alliance
The American Diabetes Association is the leading clinical authority dedicated solely to combating diabetes and its complications. Based on the latest scientific research and clinical trials, their annually updated standards of care in diabetes provides the most comprehensive and trusted evidence-based clinical guideline on the prevention, diagnosis, and treatment of diabetes and its complications.
Dr. Mike Griffiths, CEO and President of AOTI commented; “We are delighted that the ADA’s Professional Practice Committee, in its 2023 update to their standards of care in diabetes, has assessed that the now overwhelming body of clinical evidence supports awarding topical oxygen therapy a converted “A” grade recommendation as an adjunctive treatment for healing DFU.”
AOTI’s unique Topical Wound Oxygen (TWO2) therapy is unlike any other topical oxygen approach, in that it is the only device that provides a multimodality treatment, combing higher pressure oxygen delivery with non-contact cyclical compression and humidity, in a therapeutic applied by the patient at home. This patented approach has been demonstrated in numerous Randomized Controlled Trial (RCT) and Real World Evidence (RWE) studies to not only heal chronic wounds at a far higher rate, but perhaps more importantly, keep them closed longer term, thereby reducing unnecessary hospitalizations and amputations.1, 2
“The more sustainable long-term healing elicited when utilizing TWO2 therapy was highlighted in the ADA guidance, with their citing of all of the RCT and RWE studies conducted with TWO2, along with multiple recent Systematic Reviews and Meta Analyses, leading to their “A” grade recommendation ” stated Dr. Griffiths
1 Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers; The TWO2 Study. Robert G. Frykberg et al, Diabetics Care 2020; 43:616-624. https://doi.org/10.2337/dc19-0476.
2 Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes; Jessica Izhakoff Yellin, et al; Advances in Wound Care 2022; http://doi.org/10.1089/wound.2021.0118
About AOTI
AOTI is a privately-owned company based in Oceanside, California USA and Galway, Ireland that provides innovative solutions to resolve severe and chronic wounds worldwide. Our products reduce healthcare costs and improve the quality of life for patients with these debilitating conditions. Our patented non-invasive Topical Wound Oxygen (TWO2) homecare therapy is clinically proven to deliver Sustained Wound Healing that reduces both Amputations and Hospitalizations, So Life Can Get Back to Normal.
For more information see: www.aotinc.net
Contact:
Dr. Mike Griffiths
CEO & President
350571@email4pr.com
(760) 672 1920
This article was originally published here
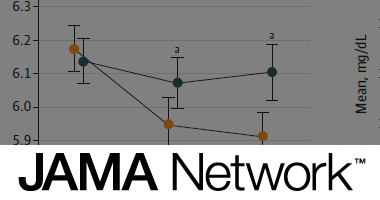
Kirsten S. Dorans, Lydia A. Bazzano, Lu Qi

Matthew Ciabattoni, DNP, FNP-BC, CWOCN Amanda C. Ward, BSN, RN-BC, CWON Ave Maria Preston, MSN, RN, CWOCN, ACNS-BC

Dr. Jeffrey Lehrman and Terrence Mabry

Author(s) : Thomas Bjarnsholt, Val Edwards-Jones, Matthew Malone, Karen Ousey, Mark Rippon, Alan Rogers, Samantha Westgate, Sabine Eming, Isabelle Fromantin, Astrid Probst, Hans Smola, Hui-Mei Yang, Jiun-Ting Yeh, Steven Percival

Mohamed S.A. Shehata Eshak Bahbah Yousef El-Ayman Ahmed H. Abdelkarim Ahmed R. Abdalla Ahmed Morshedy Khaled Turkmani Ishith Seth Nimish Seth


Elizabeth Day Dechant, BSN, RN, CWOCN, CFCN


Author(s): Jacqui Fletcher, Jaqueline Dark, Luxmi Dhoonmoon, Caroline Dowsett, Jeanette Milne, Holly Robinson, Andrew Sharpe

Joyce Black, PhD RN FAAN Elizabeth Faust, MSN, CRNP, CSWS, CWOCN-AP, MAPWCA

Rebecca Collins, Tracy Burrows, Hailey Donnelly, Peta Ellen Tehan
Schaum, Kathleen D. MS

Arturo Gonzalez, DNP, APRN, ANP-BC, CWCN-AP

Jennifer Spector, DPM, FACFAS, Assistant Editorial Director

Identification of Potential Objective Nonhealing Parameters Nicolas A. Cerusico, PhD1; Romina Chavez-Jara, PhD1; Silvana A. Lopez, MD2; Eugenia Sesto Cabral, PhD1; Aida Ben Altabef, PhD3; and Alberto N. Ramos, PhD1

Emily Greenstein, APRN, CNP, CWON-AP, FACCWS1; Maarten Vooijs, MSc2; and Sarah Norton, MSN, MBA, RN, CWCN3

Fayin Mo, Minjun Zhang, Xuewei Duan, Chuyan Lin, Duanping Sun, Tianhui You

Ilya Petrou, MD



and Diabetic Patients and Their Relatives in Saudi Arabia: A Cross-Sectional Study Sultan H. Alsaigh, Raneem H. Alzaghran, Dalal A. Alahmari, Lama N. Hameed, Kadi M. Alfurayh, Khozama B. Alaql
and Helping to Reduce Surgical Site Infections
Article is a Follow-On to MolecuLight’s Receipt an Innovative Technology Contract from Vizient Last Year
PITTSBURGH, Dec. 6, 2022 /PRNewswire/ – MolecuLight Corp., the leader in point-of-care fluorescence imaging for detection of wounds containing elevated bacterial loads, is featured in Vizient’s newly released Tech Watch publication as a key technology for visualizing bacterial load and its locations, and helping to reduce surgical site infections. The article, “Fluorescence Imaging: New technology enables point-of-care surgical wound bacterial assessment” is featured in Vizient’s Tech Watch (Medical Device) Volume 3 issue, issued this past week.
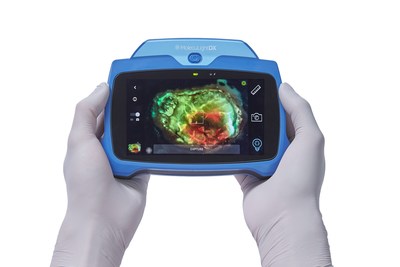
MolecuLightDX™ point-of-care Imaging device to detect elevated bacterial loads in wounds to help clinicians prevent surgical site complications. (CNW Group/MolecuLight)
The Tech Watch article describes how surgical site infections (SSIs) occur in up to 38% of surgeries1 (depending on anatomical location and type of surgery) and account for 20% of all healthcare-acquired infections2. SSIs are also the costliest of these infections, extending the average length of hospital stays by 9.7 days and costing more than $20,000 per patient admission3,4.
Early and accurate diagnosis of post-surgical bacterial loads and infection is critical to enable prompt treatment before the infection worsens. Some cases require lab testing to accurately diagnose the bacteria colonizing the wound, allowing the offending bacteria to grow and spread and delay effective treatments. Test results can take days to weeks to be available and, if positive, could be too late to prevent infection.
Clinicians need real-time diagnostic tools that they can use at the point-of-care to help provide immediate information on the state of the wound and possible growth of bacterial burden. The article argues that MolecuLight imaging helps eliminate unnecessary subjectivity in assessing wounds for the presence of harmful bacteria by allowing quick and accurate visualization of locations of elevated bacteria load in wounds, along with clinical signs and symptoms. As such, it provides “invaluable real-time information to inform clinical decision-making”.
A recent per-reviewed study5 supports this position in demonstrating the benefits of using MolecuLight to help clinician visualize bacterial burden in surgical site wounds:
“Clinicians need an objective means of detecting infection or another surgical wound complication without having to rely on subjective judgment,” says Kylie Sandy-Hodgetts, PhD, Founder and inaugural President of the International Surgical Wound Complications Advisory Panel (ISWCAP).
“Fluorescence imaging using MolecuLight is positioned to change contemporary paradigms of post-surgical wound management due to its ability to quickly and reliably detect bacterial burden and visualize contamination at the point-of-care”.
In addition to the profile in Vizent’s Tech Watch, last year the MolecuLight i:X® fluorescence wound imaging device received an Innovative Technology contract from Vizient, Inc., the nations’ largest member-driven health care performance improvement company. The new Innovative Technology contract for MolecuLight i:X signifies to Vizient members the device’s unique qualities that potentially bring improvement to the health care industry.
“We have been working with MolecuLight since 2021 when Vizient recognized the company as an awarded supplier through our Innovative Technology Program,” said Tami Maurer, VP, Contract & Program Services at Vizient, Inc. “Carefully evaluated and selected by our member council of clinical and supply chain professionals, Vizient’s Innovative Technology Program recognizes innovative advancements in care, enabling healthcare providers to offer the highest quality care while encouraging manufacturers to continue to pioneer new solutions.”
The MolecuLight i:X and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 60 peer-reviewed publications involving 1,500 patients, they are used by leading wound care facilities globally.
About MolecuLight Corp.
MolecuLight Corp. is the US subsidiary of MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight ‘s suite of commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
This article was originally published here

Tracey Rickards, BN, RN, MN, PhD Chris Boodo, MIMOSA Diagnostics

Robert Klein, DPM, FACFAS, CWS, FFPM RCPS (Glasgow)

Terasaki Institute for Biomedical Innovation



Trenton Leo, DPM; Richard Bruno, DPM, AACFAS

Wednesday, December 14, 2022 | 1:00 – 2:00pm ET

diabetic foot ulcer: a prospective, randomized, double-blind, placebo-controlled, single-center study

Christina Parker, Andrew Francis, Laura Robson and Angela Jones

Alexander Savage, Lisa J Ellis and Patricia Terrill



Aseer Manilal, Dagimawie Tadesse, Kuzhunellil Raghavanpillai Sabu


By Mia Smitt Tucson Local Media Columnist




November 20, 2022 Carrie Nagorka, Senior Editor

Kondra, Katelyn MD; Pekcan, Asli BS; Stanton, Eloise BA; Cook, Austin D. BS; Jimenez, Christian BS; Aronowitz, Alexandra BS; Winterhalter, Bridget A. PA-C, MPH; Hammoudeh, Jeffrey A. MD, DDS; Aronowitz, Joel A. MD

Last updated November 3, 2022


An interview at the SDPA 2022 Conference on major talking points from Dr. Robert Kirsner’s presentation on wound care with dermatologists and physician associates.

barriers and facilitators to foot self-care behaviors in diabetes Andrew Hill1, Mairghread Ellis, Fiona Gillison

Authors:David G Armstrong, DPM, MD, PhD Andrew J Meyr, DPM Section Editors: Russell S Berman, MD John F Eidt, MD Joseph L Mills, Sr, MD Amalia Cochran, MD, FACS, FCCM Deputy Editor:Kathryn A Collins, MD, PhD, FACS




ST. LOUIS, Nov. 15, 2022 /PRNewswire/ — Franklin W. Harry, DPM, is being recognized by Continental Who’s Who as a Trusted Podiatry Surgeon for his exemplary work in the Medical field, acknowledging his private practice achievements at Best Foot Forward.

Franklin Harry, DPM, ABMSP, is the founder of Best Foot Forward
A skilled, board-certified podiatrist, Dr. Harry is the founder of Best Foot Forward, with offices located in Festus and St. Louis, MO. With 13 years of experience practicing medicine, he enjoys working in all areas of foot and ankle care, specializing in diabetic wound care and foot and ankle surgery. His other areas of expertise include bone deformities and arthritis, and foot and ankle injuries.
Podiatry is a branch of medicine devoted to the study of diagnosis and medical and surgical treatment of various disorders of the foot, ankle, and lower extremities. A podiatrist, also known as a podiatric physician or a foot and ankle surgeon, is a medical professional devoted to treating disorders of the foot, ankle, and lower extremities. They can treat injuries and complications from ongoing health issues like diabetes.
Best Foot Forward (BFF) is committed to providing excellent podiatric care by enhancing the quality of life of patients with foot, ankle, and leg problems. BFF strives to preserve and restore the health of the lower extremities and provide patient-centered care. Their podiatrists treat everything from difficult to trim toenails, calluses, warts, foot infections, diabetic wound care, fall prevention, and surgical foot and ankle repairs.
Before starting his medical career, Dr. Harry earned his Doctor of Podiatric Medicine degree from Barry University in Miami, FL. He completed his surgical residency at Wyckoff Heights Medical Center and received advanced training with the Central Kentucky Diabetes Fellowship, specializing in complex wounds, limb salvage, and the biomechanics of fall prevention.
An authority in his field, the doctor is a member of the American Association of Podiatric Practice Management and is board-certified by the American Board of Multiple Specialties in Podiatry. He is a member of the American Board of Podiatry, the Relias Wound Care Institute, the Missouri Podiatric Medical Association, and the Illinois Podiatric Medical Association.
Outside of his practice, the doctor volunteers with Peter and Paul and Biddel House homeless shelters, providing podiatric medical care for the less fortunate. In addition, the doctor goes on mission trips to Haiti with Hands Helping Haiti and to Guatemala with Washington University.
In his free time, Dr. Harry enjoys spending time with his wife, Misty Gonzalez, Pharm. D., to whom he has been married since 2017. They have one child and a dog named Bentley. They enjoy cooking, hiking, scuba diving, exploring new restaurants, and are avid sports fans.
In light of this recognition, Dr. Harry wishes to thank his mentors: Ronald Guberman, DPM, Jonathan Moore, DPM, and Pamela Jensen, DPM.
For more information, visit www.bffdocs.com.
This article was originally published here

Nancy Collins, PhD, RDN, LD, NWCC, FAND

By Barret Halgas, MD

HIGH WYCOMBE, England, Nov. 9, 2022 /PRNewswire/ — Firstkind Ltd, innovator and manufacturer of the clinically proven geko™ device – a ground-breaking advanced therapy for chronic venous leg ulcer healing – is seeking senior tissue viability nurses, willing to embrace and drive innovation advance, to join its Partner-With-Us program.
The geko™ device on the leg
The Partner-With-Us program precedes the imminent publication of a statistically significant randomised controlled study that compares the rate of wound healing in chronic venous leg ulcer patients receiving the geko™ device as an adjunct to compression therapy, compared to compression therapy alone.
A transformative innovation, the geko™ device is a small, self-adhesive, wearable neuromuscular electro-stimulator (NMES) that is applied to the skin just below the knee, over the head of the fibula. It delivers a gentle intermittent electrical pulse, once per second, to the common peroneal nerve. This elicits a muscular twitch that activates the calf and foot muscle pumps, increasing venous, arterial, and microvascular blood flow – transporting oxygenated blood to the wound bed and edge to promote wound healing.
In addition to the benefit of better patient outcomes and the release of significant cost savings for primary care, TVNs keen to join the Partner-With-Us program – to drive innovation advance – will gain:
If you would like to know more about the Partner-With-Us program, and what the geko™ device can do for your patients, please email: Fiona.Young@firstkindmedical.com. We look forward to hearing from you soon.
About Firstkind Ltd (a Sky Medical Technology Company)
Firstkind Ltd is a UK-based medical devices company. Through its innovative mechanism of non-invasive neuromuscular electrostimulation (NMES), Firstkind has developed a ground-breaking NMES technology platform, OnPulse™, embedded in its industry-leading product, the geko™ device. The company develops a range of products tailored to the needs of different medical application areas, selling both direct and through strategic partnerships or distributors in each major clinical area. Clinical areas of focus include chronic wound healing, the treatment and prevention of oedema (swelling) and venous thromboembolism prevention (VTE). The goal in each therapy is to partner with healthcare professionals to improve clinical outcomes and patient care while at the same time reducing costs for health systems.
This article was originally published here

Dr. Tracey Rickards, BN, RN, MN, PhD Associate Professor, Faculty of Nursing, University of New Brunswick


Jennifer Spector, DPM, FACFAS, Assistant Editorial Director
SIERRA MADRE, Calif., Nov. 10, 2022 /PRNewswire/ — Our doctors, nurses, and clinicians have been at the front lines of treating the consequences of diabetes every day. With over 20 years of supporting wound centers, we’ve learned 2 important truth’s about diabetes:
Diabetes continues to grow at an alarming rate
More action and education are needed

Steal this Diabetes Month Resource Kit: thewca.com/2022/11/01/steal-this-diabetes-month-resource-kit/
To commemorate November’s National Diabetes Awareness Month, Wound Care Advantage does not want to focus on the statistics of Diabetes. Like the 37 million people suffering from diabetes, or the fact that up to 34% of those patients will develop a foot ulcer (DFU) in their lifetime, and that DFUs are the number one leading cause of non-traumatic amputations. Instead, we realize that diabetes is not going anywhere anytime soon, nor is the knowledge that if not managed properly, diabetes can lead to serious and fatal outcomes.
This is why this November, WCA is giving Wound Programs a Diabetes Month Resource Kit to build awareness of diabetes and the high risk of chronic wounds. With 70% of diabetic foot ulcers ending in amputation and leading to a 2-year life expectancy after surgery, wound care programs deserve the support and resources to save the limbs and lives of all patients. Help us put a spotlight on this disease with our free Diabetes Month Resource Kit. Steal our professional resources for your clinic.
Our Resource Kit will supply you with:
Even with the disease of diabetes keeping its alarmingly quick growth rate, the population of patients that develop an ulcer that leads to an amputation does not have to. Join us in building the awareness that 70% of DFU patients do not need to end with an amputation this November.
Diabetes Month Resource Kit: thewca.com/2022/11/01/steal-this-diabetes-month-resource-kit/
About Wound Care Advantage:
Founded in 2002, Wound Care Advantage (WCA) has been supporting wound centers for 20 years. With a strong commitment to care and innovation, WCA advocates for the financial independence of partner hospitals and the rapid healing of patients they serve. Wound Care Advantage is a privately held company headquartered in Sierra Madre, California. For additional information, visit www.thewca.com.
SOURCE Wound Care Advantage

Roberto Cassino, MD, Sacra Famiglia Korian Nursing Home, Italy

Tuesday, November 29, 2022 | 2:00 – 3:00pm ET Daniel L. Kapp MD



dehydrated human amnion chorion membrane for use in pressure injuries Michael Sabolinski, MD, Sabolinski LLC, Franklin, MA

Alik Farber, Matt Menard and Ken Rosenfeld


Margaret Hiler MSN, RN, CWOCN
The North American Center for Continuing Medical Education, LLC (NACCME), the CE-sponsor for the Symposium on Advanced Wound Care (SAWC) Spring and Fall meetings, and SAWC co-host, HMP Communications, LLC, today announced the inaugural class of five SAWC Spring scholarship winners, selected in cooperation with several prominent wound care societies and associations.
The SAWC Spring and SAWC Fall meetings are the largest wound care conferences in the United States with 2,000+ attendees expected at the 25th annual SAWC Spring and Wound Healing Society (WHS) Meeting to be held, April 19 -22, 2012 in Atlanta, Georgia. Wound care is predominately a multidisciplinary focused practice with optimal outcomes often provided by a team including physicians, podiatrists, therapists and nurses. The SAWC Spring scholarships not only target these clinical specialties but wound care fellows and researchers as well.
The owners of SAWC Spring have reached out to several major wound care societies and associations, along with key opinion leaders, to choose the inaugural 2012 SAWC Spring class of honorees. The five SAWC Spring scholarship winners will be honored during the annual SAWC Spring VIP Party to be held on Thursday night, April 19, 2012, at the Terraces Restaurant in the Georgia World Congress Center. Each of the scholarship winners will receive complimentary registration to SAWC Spring/WHS along with a framed certificate presented by their sponsor during the ceremony.
The inaugural list of SAWC Spring scholarship winners at the 2012 SAWC Spring/WHS Conference includes:
“Wound Healing Fellow Scholarship” – Malgorzata Plummer, MD, Assistant Professor of Clinical Surgery, Section of Wound Healing and Tissue Repair, University of Illinois at Chicago,
Presenter – William Ennis, DO, MBA, FACOS, President, American College of Wound Healing and Tissue Repair (ACWHTR)
“Wound Care Nursing Scholarship” – Sue Girolami, RN, BSN, CWOCN, Clinical Manager, Therapy Support, Inc.
Presenter – Terry Treadwell, MD, FACS, President, Association for the Advancement of Wound Care (AAWC)
“Wound Healing Research Scholarship” – Kenneth Finnson, PhD, Research Associate, Montreal General Hospital, McGill University Surgical Research
Presenter – Harriet Hopf, MD, President, Wound Healing Society (WHS)
“Wound Care Therapist Scholarship” – Jaimee Haan, PT, CWS, Team Leader – Physical Therapy Wound Management Department, University of Indiana Health
Presenter – Rose Hamm, DPT, CWS, President – American Physical Therapy Association (APTA) Wound Management Special Interest Group (WMSIG)
“Wound Care Training Scholarship” – Jeffrey S. Danetz, MD, FACS, Medical Director, Edward White Hospital Wound Center and Largo Medical Center Wound Center
Presenter – Robert Kirsner, MD, PhD, Co-Chairperson, SAWC
Recognizing the importance of appropriate and timely wound care in high-risk patient populations, and in concert with SAWC Spring/WHS, Georgia Gov. Nathan Deal has proclaimed April 2012 to be “Chronic Wound Care Month.”
For more information on the 25th Annual SAWC Spring/WHS meeting being held in April 19–22, 2012 in Atlanta, GA, please visit http://spring.sawc.net/ or contact Tiffney Oliver at 609-630-6223.
About NACCME
The North American Center for Continuing Medical Education, LLC (NACCME), an HMP Communications Holdings Company, provides the highest quality CME/CE across medical disciplines and therapeutic areas. In conjunction with top medical faculty, NACCME develops evidence-based initiatives that target specific educational needs, assisting healthcare professionals in improving patient outcomes by bridging the gap between current and best healthcare practices.
About HMP Communications, LLC
HMP Communications, LLC (HMP), is a leader in healthcare communications and education. It publishes some of the nation’s most well-respected journals in three key medical arenas — wound care/podiatry, cardiovascular and life sciences — representing 14 specialties. HMP also offers more than 20 years of meeting management/production expertise and over a decade producing cutting-edge, online educational programs. HMP’s portfolio of wound care/podiatry journals includes; Ostomy Wound Management, WOUNDS, Podiatry Today and Today’s Wound Clinic.
This article was originally published here

the Concerning Trend Involving Skin Pigmentation and a Role for Fluorescence Imaging

by Caroline Fife, M.D. from Oct 11, 2022

Lilibeth Acero, BS, RN, DAPWCA

Treatment Outcomes at a Tertiary Hospital in South-Western Uganda Authors Sikhondze MM , Twesigye D, Odongo CN , Mutiibwa D, Tayebwa E, Tibaijuka L , Ayana SD, Cabrera Dreque C

by Navin Kumar Verma, Nanyang Technological University Singapore

Abeona hopes to submit FDA application for therapy’s approval by mid-2023 by Lindsey Shapiro, PhD

of Type 2 Diabetes Mellitus Patients Associated with Diabetic Foot Ulcer


with Its Patented Regenerative Solution D’OXYVA with Recovered CO2

SIERRA MADRE, Calif., Oct. 27, 2022 /PRNewswire/ — Delayed wound care can mean a limb or a life, many times both, for a patient with a non-healing wound. With the rate of patients living with a chronic wound nearing the 7 million mark in the United States, and 2 million of those suffering from a diabetic foot ulcer, the need for advanced wound care is greater than ever.

Wound care is vital for patients and their communities. Keeping every center open and financially viable has been our mission for 20 years. Today we give back some of that wisdom, knowledge, and experience from 20 years of supporting wound centers. This free crash course is for hospital leaders, future leaders, or anyone interested in wound care. 🎬 Watch Now: thewca.com/crash-course
However, in the midst of this “silent” epidemic, wound care programs are finding themselves having to fight to keep their doors open. And when they are open, many are without the support they deserve. Which is why keeping every center open and financially viable has been our mission for the last 20 years. Within these trying times, we want to offer more than just words of wisdom with our “Steal This” series available to all wound care programs throughout the nation.

Steal this Crash Course. 🎬 Watch our crash course for free: thewca.com/crash-course
“Steal This” is exactly what it sounds like. We want all programs to steal our ready-made resources and wound care education to use immediately. This is in hopes to help ease some of the stress wound care programs are facing in today’s world, and be a figure of support to all in the industry.
The first “Steal This” will be the release of the Program Leadership Crash Course series. In this free course for industry leaders, we help navigate the challenges of day to day tasks, and will cover all aspects of running a successful program. Along with each topic is a supplemental resource book that includes need to know information, questions you should be asking yourself, and action steps to do today. Steal it, use it, and heal more wounds with the Program Leadership crash course where we give back the wisdom, knowledge, and experience we’ve gained through-out the 20 years of supporting wound centers.
For more information about Crash Course, please visit www.thewca.com/crash-course
About Wound Care Advantage:
Founded in 2002, Wound Care Advantage (WCA) has been supporting wound centers for 20 years. With a strong commitment to care and innovation, WCA advocates for the financial independence of partner hospitals and the rapid healing of patients they serve. Wound Care Advantage is a privately held company headquartered in Sierra Madre, California. For additional information, visit www.thewca.com.
SOURCE Wound Care Advantage
This article was originally published here

Keith Loria

Jesse Obregon, Lauren DeLamielleure, Taha F. Rasul, Brittany Blake, Armen Henderson

John C. Smith, Marco Comianos, Wesley Tang, Satish Sarvepalli




Abigail Chaffin, MD

JACKSONVILLE, Fla., Nov. 1, 2022 /PRNewswire/ –As millions of Americans living with diabetes are also living with chronic wounds that won’t heal, Healogics® is raising awareness of diabetes-related wounds as part of the Healogics ninth annual Diabetes Awareness Campaign.
Throughout November, Wound Care Centers® will educate the local community about the importance of awareness, early intervention and specialized care for diabetes-related chronic wounds, like diabetic foot ulcers. Local team members will also visit healthcare providers in surrounding areas to provide important information to help at-risk patients living with diabetes.

Diabetes Awareness Infographic
There are more than 37 million Americans currently living with diabetes, according to the American Diabetes Association (ADA). Additionally, there are 96 million American adults who have prediabetes, leading to 1.4 million new diagnoses of diabetes every year. Diabetes-related wounds are a leading cause of limb loss, accounting for nearly 70 percent of cases undergoing lower extremity amputation in the United States.
“This campaign is essential because early detection of diabetes-related wounds significantly reduces amputation risks. Diabetic foot ulcers are the leading cause of diabetes-related hospitalizations and lower-limb amputations. What starts as a small cut or blister can quickly progress into a non-healing wound with severe complications. With 50 percent of our patient population living with diabetes, we know firsthand that our awareness efforts can help improve the lives of those struggling with diabetes-related wounds,” said Healogics Chief Executive Officer Frank Williams.
Many suffering from chronic wounds have been negatively affected by the COVID-19 pandemic as they have eschewed needed care during the past two-plus years. Untreated and undertreated wounds have resulted in amputation, according to a study from the ADA. Of the patients who have undergone one amputation, 55 percent will require amputation on the second leg. An amputation results in decreased quality of life, increased medical costs and a significantly higher risk of mortality.
“Many people who come to the Wound Care Center® with chronic wounds are among the 37 million adults living with diabetes. Some were unaware that diabetes put them at greater risk for non-healing wounds. Encourage patients to check their feet every day. It’s imperative we help patients avoid the serious consequences of non-healing wounds, such as diabetic foot ulcers, by raising awareness of the risks and importance of daily foot screenings to help prevent an avoidable amputation,” said Healogics Chief Medical Officer Dr. William Ennis.
Factors that may increase the risks of developing a chronic wound, such as a diabetic foot ulcer, include high blood sugar levels, poor circulation, immune system issues and nerve damage. Risk factors for diabetes include age, diet, activity level, obesity and heredity.
Healogics recommends the following to help prevent diabetic foot ulcers:
Early detection and specialized care from a Wound Care Center® can reduce healing times and significantly reduce the risk of amputation.
Contact Healogics to learn more about diabetic foot ulcers or if you have a wound that will not heal. To schedule an appointment, please call 1-800-379-9774 or visit Healogics.com.
About Healogics
Headquartered in Jacksonville, Fla., Healogics is the nation’s wound healing expert. Last year over 300,000 patients received advanced wound care through a network of over 600 Wound Care Centers. Healogics also partners with over 300 skilled nursing facilities to care for patients with chronic wounds and provides inpatient consults at more than 60 partner hospitals. As the industry leader, Healogics has the largest repository of chronic wound-specific patient data in the country. The Healogics Wound Science Initiative offers peer-reviewed research and advanced analytics in the pursuit of not only better outcomes, but a better way to provide care.
SOURCE Healogics, LLC
This article was originally published here


David G. Armstrong, DPM, MD, PhD




Nancy Collins, PhD, RDN, LD, NWCC, FAND
A Randomized Controlled Trial

Two leading wound industry solutions companies, with a shared passion for improving patient outcomes, are collaborating to improve the way that Home Health and other in-home providers care for patients with chronic wounds.
SAVANNAH, GA. (PRWEB) OCTOBER 24, 2022
HARTMANN USA and Corstrata announce their collaboration to support home health and other in-home providers in caring for wound patients at a time when the incidence of complex chronic wounds is increasing while access to wound care nurse specialists is becoming more challenging. At the core of a successful wound care program is access to both highly effective advanced wound dressings as well as clinical expertise to implement evidence-based treatment protocols and monitor wounds to closure. According to a recent study published by top-50 accounting firm BerryDunn, National Healthcare at Home Best Practices and Future Insights Study, 100% of Home Health Centers of Excellence (those Home Health agencies in the top 10% for quality and patient satisfaction and with a positive financial surplus) have a wound-certified specialist on staff.
This collaboration will increase access to Corstrata’s team of virtual board-certified wound nurses (WOC nurses) and HARTMANN’s suite of advanced wound care products to improve clinical and financial outcomes for in-home providers that care for patients with chronic wounds.
HARTMANN has been providing advanced wound dressing solutions globally for over 150 years and has evolved with its broad portfolio of high-quality, cost-effective products that provide home health clinicians with a simplified, consistent approach for effectively managing wounds. Corstrata provides virtual wound and ostomy care management nationwide across multiple provider settings, including home health, skilled nursing facilities, hospice, and emerging hospital-at-home solutions companies.
According to Jon Procopio, Managing Director of HARTMANN USA, “Patient care is our priority. HARTMANN strives to enable the progression of the wound towards complete healing that patients deserve and strengthen the confidence that healthcare professionals need to provide wound care. We have a nationwide team of dedicated account and customer care representatives specifically trained for consultation, education, and support in offering clinical and business solutions. Now, with Corstrata, we will enhance access to clinical expertise related to wound care through their team of certified WOC nurses.”
“The Corstrata team is excited about this important collaboration with HARTMANN to create access to Corstrata’s virtual WOC nurses for customers and the patients they serve. With up to one-third of all home health and hospice patients having a chronic wound, it is critical for clinicians to provide evidence-based care to both prevent and heal wounds,” says Joseph Ebberwein, co-founder and Chief Financial Officer of Corstrata. “At this time when agencies are struggling with critical staffing shortages, including WOC nurses, and increasing financial challenges, having a strong wound program is essential. This collaboration between Corstrata and HARTMANN provides a path to success.”
According to Katherine Piette, Corstrata’s CEO, the decision to collaborate with HARTMANN is an easy one. “Our virtual WOC nurses rely on our provider customers having access to highly effective advanced wound dressings to accelerate wound healing and reduce the overall cost of patient care, ” Piette says. “HARTMANN has a unique suite of advanced dressings that are being used by some of the top home health providers in the U.S. with impressive results. We are excited about the opportunity to improve the level of wound care provided for this ever-growing cohort of complex wound patients. Our clients can access clinical support from Corstrata when needed without the cost of hiring their own WOC nurse, a costly and often frustrating proposition. This collaboration will equip providers with turn-key wound solutions that they have been missing in their clinical care delivery at a crucial time in the industry.”
About HARTMANN
The HARTMANN GROUP is one of the leading providers of wound treatment and skin integrity solutions around the world. Wound dressings and maintaining healthy skin have been at the heart of HARTMANN from the beginning when we introduced the world’s first antiseptic wound dressing over 150 years ago. Overall, HARTMANN looks at rich legacy. Every day, healthcare professionals and patients rely on HARTMANN brands in the segments of Incontinence Management (e.g. MoliCare®), Wound Care (e.g. Zetuvit®) and Infection Management (e. g. Sterillium®). This is expressed in our brand promise of “Helps. Cares. Protects.” In 2021, the HARTMANN GROUP reported Group sales of EUR 2.3 billion.
For the latest information on HARTMANN, follow @HARTMANN_GROUP on Twitter.
To learn more about the HARTMANN GROUP, click here.
To learn more about HARTMANN USA, click here.
About Corstrata
Corstrata is a virtual care solution that utilizes technology to provide access to scarce certified wound and ostomy nurses at the patient’s bedside in post-acute provider settings, including home health, hospice, skilled nursing facilities, and emerging hospital-at-home providers. Corstrata’s team of WOC nurses provides consultations with provider staff at the patient’s bedside, either through HIPAA-compliant video or through review of store-and-forward wound images, to improve clinical and financial outcomes for providers.
For the latest information on Corstrata, follow @Corstrata on Twitter.
To learn more about Corstrata, click here.
This article was originally published here
From Feb 7, 2022 – David Armstrong, Desmond Bell, Rick Zollinger, Randy Cook, Rhonda Crowe

Liqiong Yuan 1, Maoting Ye, Ting Yang


Negin Shamsian, Heather Hettrick, Adriano Mehl, Tobe Madu, Mark Barakat, Windy Cole, Michael Oliver

Schaum, Kathleen D. MS

Pasek, Jarosław MD, PhD; Stanek, Agata MD, PhD; Szyluk, Karol MD, PhD; Cieślar, Grzegorz MD, PhD
major differences in immune response-modulatory effects


Fay Crawford, Donald J. Nicolson, Aparna E. Amanna & Marie Smith

A Prospective Comparative Study – Jamuna Nagaraj, Venkatesh Subbiah

Audrey Abella
Indre Jasineviciute, Juozas Grigas, Gintare Ziukaite, Arnoldas Pautienius, Dainius Razukevicius, Judita Zymantiene & Arunas Stankevicius


Wed, Nov 2, 2022 · 2:30 PM Eastern Time (US & Canada) (GMT -4:00)

ADVANCED WOUND CARE SUMMIT


Muñoz, Victoria; Pino, Ander; Martinez, Carmen; Echevarria, Begoña; Lacramioara, Varlan; Anitua, Eduardo


Neil Baker OBE

Jennifer Spector, DPM, FACFAS, Assistant Editorial Director

A Case Study Pérez-Acevedo, Gemma; Bosch-Alcaraz, Alejandro; Torra-Bou, Joan Enric

Jennifer Spector, DPM, FACFAS, Assistant Editorial Director



Sylvie Hampton

A Multiple Case Series

Dr. Abigail Chaffin
surpassing 2,000 peer-reviewed medical journal studies published
ST. PAUL, Minn., Oct. 13, 2022 /PRNewswire/ — 3M Health Care’s Medical Solutions Division today announced its 3M™ V.A.C.® Therapy negative pressure wound therapy (NPWT) has surpassed a clinical evidence milestone of 2,000 published, peer-reviewed medical journal studies. V.A.C. Therapy is the first and only NPWT solution to garner this number of published studies about its therapy. It is backed by more clinical data than any other brand, accounting for more than 75% of published NPWT clinical evidence.
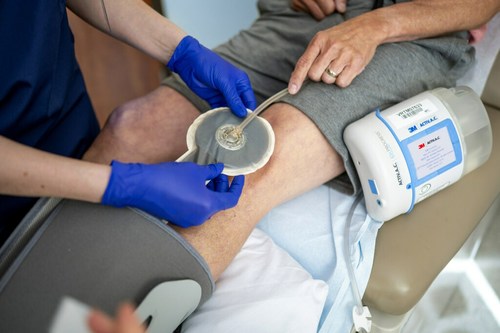
3M™ V.A.C.® Therapy negative pressure wound therapy (NPWT) has surpassed a clinical evidence milestone of 2,000 published, peer-reviewed medical journal studies. It is backed by more clinical data than any other brand, accounting for more than 75% of published NPWT clinical evidence.
The clinical studies have been conducted by wound care professionals worldwide and published in journals across the globe, covering a comprehensive range of wound types, wound care settings and study formats, such as case studies, economic studies, randomized controlled trials and more.
“Clinical evidence has always been a foundational element to establishing credibility for V.A.C. Therapy and our NPWT products in the wound care community,” said Ronald Silverman, M.D., 3M Health Care senior vice president of clinical affairs and chief medical officer. “Published studies have also helped to promote adoption of NPWT and spur therapy innovations, including 3M™ Prevena™ Therapy for incision management, 3M™ Veraflo™ Therapy for instillation therapy for open wounds, 3M™ AbThera™ Open Abdomen Negative Pressure Therapy. Our team members in the field are also actively engaged with wound care experts worldwide, working right alongside clinicians to observe the changing nature of wound care and gather feedback about our products, which helps us identify opportunities for innovation.”
Today, V.A.C. Therapy is used across a spectrum of health care settings, from acute care facilities to ambulatory surgical centers, assisted living facilities, and in patients’ homes. In the U.S., V.A.C. Therapy is available with 24/7 remote therapy monitoring to support adherence to the therapy. 3M’s NPWT portfolio continuously evolves to meet clinician and patient needs. Last year, 3M launched the first-ever silicone-acrylic hybrid drape for use with V.A.C. Therapy, the 3M™ Dermatac™ Drape, an innovation designed to be gentle on patients’ skin and easy for clinicians to use.
“Today’s wound care patients are often sicker and have more comorbidities, making their wounds more complex to treat and increasing the demands on clinicians’ time. 3M strives to provide a robust tool selection to address clinicians’ unique wound care needs and make our products easier to use to help save their valuable time — and ultimately, help transform outcomes and improve lives for wound care patients,” said Dr. Silverman.
For more information, visit www.3m.com/npwt.
About 3M
3M (NYSE: MMM) believes science helps create a brighter world for everyone. By unlocking the power of people, ideas and science to reimagine what’s possible, our global team uniquely addresses the opportunities and challenges of our customers, communities, and planet. Learn how we’re working to improve lives and make what’s next at 3M.com/news or on Twitter at @3M or @3MNews.
Photo – https://mma.prnewswire.com/media/1919999/3M_VAC_Therapy.jpg
Logo – https://mma.prnewswire.com/media/1343410/3M_Logo.jpg
SOURCE 3M
This article was originally published here


Michael King, Chief Medical Officer, Upperline Health
Studies of Wound Closure Rate with Novel Chronic Wound Treatment Continue
SARASOTA, Fla., Oct. 13, 2022 /PRNewswire/ — Omeza today announced that the Centers for Medicare and Medicaid Services (CMS) has confirmed a HCPCS reimbursement code for Omeza® Collagen Matrix; code A2014, “Omeza collagen matrix, per 100 mg” was established to describe the product.

Omeza® Collagen Matrix is the first of its kind drug-device combination product, with a simple snap and squeeze application for chronic wounds.
Omeza® Collagen Matrix is the first of its kind drug-device combination product, with a simple snap and squeeze application for chronic wounds. The FDA-cleared drug-device received a Level II Healthcare Common Procedure Coding System (HCPCS) reimbursement code paving the way for providers to receive reimbursement from Medicare.
The decision came after application was made to CMS in late 2021. Omeza Chief Commercial Santino Costanza stated, “This decision by CMS opens the doors to Omeza’s innovative treatment line for millions of Americans covered by Medicare who are currently suffering from chronic wounds. Now we look forward to educating commercial payors on the health, financial and humanitarian benefits of a positive reimbursement decision.”
Earlier this year the Department of Veterans Affairs Federal Supply Schedule (FSS) Service granted contract status for Omeza® Collagen Matrix. All Omeza products are available to government agencies through Marathon Medical, a prime vendor for the VA.
Currently, three US clinical trials are investigating the use of the three-product Omeza treatment product line, which includes Omeza® Collagen Matrix, for documentation of healing rates in venous ulcers, diabetic ulcers, and other chronic wounds. Concurrently, individual case studies submitted by providers testing the Omeza treatment product line on chronic wound closure in their clinical settings report an average percentage area reduction (PAR) of 60% at 4 weeks.
Omeza ® Collagen Matrix is indicated for the management of wounds including partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds (donor sites/grafts, post-Moh’s surgery, post-laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, superficial partial thickness burns, skin tears) and draining wounds.
Omeza ® Collagen Matrix is the first drug/device combination product to deliver an anhydrous 3- dimensional microstructure of collagen to challenging wounds. When applied to a wound surface, the snap and squeeze matrix is naturally incorporated into the wound over time. Omeza® Collagen Matrix is designed for intimate contact with both regular and irregular wound beds, to provide a conducive environment for the patient’s natural wound healing process.
About Omeza:
Omeza (www.omeza.com) is a skin science company pursuing equitable access to better wound care outcomes for patients at all sites of care. The company is based in Sarasota, FL USA. Inquiries from medical and health professionals should be directed to info@omezapro.com.
SOURCE Omeza LLC
This article was originally published here



aimed at regulated health professionals in Ontario

Nancy Collins, PhD, RDN, LD, NWCC, FAND Giovanna Rosario Arroyo, MA, RDN, CD, LDN




2022 Fall Symposium on Advanced Wound Care (SAWC)

NEWS PROVIDED BY
American College of Wound Healing and Tissue Repair
Oct 11, 2022, 09:00 ET
American Society of Plastic Surgeons and American College of Wound Healing and Tissue Repair to Discuss Fellowship Training to Improve Patient Outcomes
BOSTON, Oct. 11, 2022 /PRNewswire/ — The American Society of Plastic Surgery (ASPS) and the American College of Wound Healing and Tissue Repair (ACWHTR) will hold a joint strategic planning meeting on Oct. 27 in Boston to discuss advanced fellowship training in wound healing and tissue repair.
Since 2011, ACWHTR has trained fellows in wound healing and tissue repair at the University of Illinois at Chicago and helped create similar university-based programs that focus on training non-surgeons in the field of wound healing.
“With more than 6.2 million people in the United States living with non-healing wounds – magnified by an aging society as well as epidemics of obesity and diabetes, the number of patients with these conditions continues to grow,” says ASPS President J. Peter Rubin, MD, MBA. “Over the past year, ASPS and ACWHTR have engaged in dialogue about establishing new training paradigms for this much-needed and constantly evolving clinical field.”
Non-surgical providers lead many wound care centers; however, surgeons who provide the needed surgical procedures are also increasingly taking on leadership roles as medical directors, in-patient service chiefs, and leaders in the field.
This strategic planning session is open to all interested providers, medical and surgical professional society representatives, and those currently directing surgical, non-surgical or hybrid training programs. The goal of the meeting is to improve patient outcomes across many settings by establishing a formal, consistent educational curriculum. ASPS has led the process and proposes a non-ACGME match program for plastic surgery residents to build on the trainee’s foundational knowledge in skin and wound care procedures.
“A further objective of the collaboration is to increase the number of non-surgical fellowships based on the current ACWHTR educational platform,” says president and founder of ACWHTR, William J. Ennis. DO, MBA.
The strategic planning and ASPS/ACWHTR Wound Care Fellowship launch meeting will take place at 1:15 p.m. EDT at the Boston Convention & Exhibition Center on Oct. 27, during Plastic Surgery The Meeting in Boston. The option to participate virtually is available. Kindly respond to ASPS Senior Vice President Gina T. McClure at gmcclure@plasticsurgery.org for additional information or to register for the virtual event.
About American College of Wound Healing and Tissue Repair
The American College of Wound Healing and Tissue Repair (ACWH) is a 501(c)(3) nonprofit organization based in Chicago that is committed to advancing the field of wound care through education, research, and advocacy. The College fosters the training of medical professionals through the sharing of a physician-based, clinical fellowship curriculum developed in conjunction with the University of Illinois Hospital and Health Sciences System and allied healthcare colleges and programs. The goal of the organization is to designate wound care as a board-certified medical specialty.
About the American Society of Plastic Surgeons
The American Society of Plastic Surgeons (ASPS) is the largest organization of board-certified plastic surgeons in the world. Representing more than 7,000 physician members, the society is recognized as a leading authority and information source on cosmetic and reconstructive plastic surgery. ASPS comprises more than 93 percent of all board-certified plastic surgeons in the United States. Founded in 1931, the society represents physicians certified by The American Board of Plastic Surgery or The Royal College of Physicians and Surgeons of Canada.
SOURCE American College of Wound Healing and Tissue Repair
This article was originally published here
FREDERICK, MD. (PRWEB) OCTOBER 11, 2022
Nearly 6 million people in the U.S. suffer with chronic wounds. That care translates to more than $96.8 billion in annual Medicare costs. Nanobiofab, a nanotechnology startup founded by Dr. Xiaonao Liu, is working to make detection and care of such wounds quicker and more efficient by utilizing their proprietary real-time agnostic patch for infection detection, an intelligent nose known as RAPID-iNose.
The company is a member of the Frederick Innovative Technology Center, Inc. and was recently awarded a $250,000 Defense Health Agency SBIR Program contract for their proposal, “RAPID-iNose: Real-time Agnostic Patch for Infection Detection using Intelligent Nose.” The project combines a patented, wireless and highly-sensitive nanosensor array with artificial intelligence algorithms. In tests to date, the RAPID-iNose automatically and continuously captures information on the types and amounts of pathogens. Results can then prompt infection alerts to patients and doctors, eliminating some of the biggest challenges in wound care.
Dr. Liu explains, “Currently, clinical judgment is required for diagnosis and treatment. However, symptoms of wound infection are commonly masked in patients with complex wounds.”
Considered the current “gold standard,” culture-based antimicrobial susceptibility testing is now conducted under aseptic conditions in laboratories. This requires assessment by specialists using reagents, which may not be logistically feasible, especially in a battlefield environment. Turnaround is also slow, taking anywhere from hours to days for processing, depending upon transportation of the sample. Caregivers lack the means for continuous sampling to monitor patient progress. And there is no Food and Drug Administration approved, telemedicine-capable, deployable device for early detection of wound infections or real-time monitoring in either field environments or hospital settings.
Nanobiofab’s previous work with the Small Business Innovation Research Program includes efforts to improve medical training through AI-enhanced clinical simulators with West Virginia University’s Center for Simulation Training and Education for Patient Safety. Kathie Callahan Brady, Nanobiofab advisor and FITCI CEO, says, “This company creates revolutionary million-scale nanomaterial that can be used in wide-ranging applications, from routine health monitoring to cancer detection, and everyone is an opportunity to improve the lives and reduce suffering.”
RAPID-iNose will be the world’s first wearable intelligent device for real-time monitoring of wound infection, replacing current resource-intensive detection methods. The device has the potential to help clinicians identify pathogens quickly, safely and easily, allowing for effective use of antimicrobials as well as reducing treatment costs substantially.
For more information on Nanobiofab or their nanosensor research, log on to http://www.nanobiofab.com.
This material is based upon work funded by the Defense Health Agency Small Business Innovation Research /Small Business Technology Transfer Programs under US Army Medical Research Acquisition Activity.
Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Department of Defense, DHA SBIR/STTR Programs or USAMRAA.
This article was originally published here


Technique in the Closure of Open Fracture Wound and Infected Wound With Significant Skin Loss