of Detecting Bacterial Burden in Surgical Site Wounds by 11-Fold
Authors Suggest that Fluorescence Imaging of Bacterial Burden is Positioned to Change
Contemporary Paradigms of Post-Surgical Wound Management
TORONTO, Jan. 18, 2022 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging for real-time detection of wounds containing elevated bacterial loads, announced the publication of “Uncovering the high prevalence of bacterial burden in surgical site wounds with point-of-care fluorescence imaging“1 in International Wound Journal. The publication reports on the results of an analysis of 58 imaged and biopsied surgical site wounds from the 350-patient multi-centre FLAAG (fluorescence imaging assessment and guidance) clinical trial2.
Key findings of the study include:
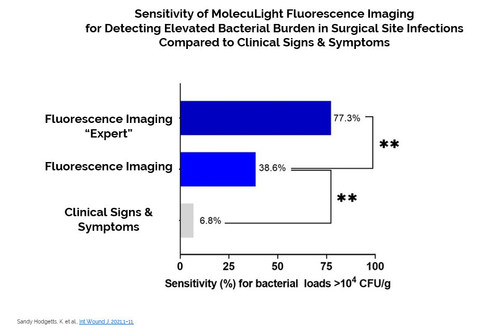
- 76% of surgical sites that reach the stage of referral to a wound specialist had clinically significant bacterial loads (104 to 109 CFU/g), however only 6.8% exhibited symptoms of infection, resulting in delayed infection management.
- Point-of-care fluorescence imaging (using the MolecuLight i:X device) for detecting high bacterial loads improved sensitivity by 5.7-fold compared to clinical signs and symptoms alone.
- Clinician experience with fluorescence imaging and interpretation (>200 imaging sessions) increased sensitivity of fluorescence imaging to 11.3-fold higher than clinical signs and symptoms alone, and accuracy to 2.6-fold higher.
The incidence of surgical wound complications, including surgical site infections (“SSI”), continue to rise and the development of an SSI is associated with a marked increase in morbidity, a 2-to 11-fold increase in mortality rate, and prolonged hospital stays3. Approximately 2-5% of surgical wounds in the US develop an SSI7-10 at an annual cost of up to $10 billion4-7. This includes extended hospital stays, readmissions, and more resources required to manage complications.
“While early identification and management of high bacterial burden is critical for the prevention of surgical site infections, this study shows that pathogenic bacterial burden is present in most (>75%) surgical wounds that are referred to a wound specialist, but is largely asymptomatic and therefore goes undetected, delaying bacterial management strategies”, says lead author Associate Professor Sandy-Hodgetts, Centre for Molecular Medicine & Innovative Therapeutics, Murdoch University & Senior Research Fellow, School of Biomedical Sciences, University of Western Australia and the Founder and inaugural President of the International Surgical Wound Complications Advisory Panel (ISWCAP). “Due to its ability to quickly and reliably detect bacterial burden at the point-of-care, fluorescence imaging using the MolecuLight device is positioned to change contemporary paradigms of post-surgical wound management”.
These findings are part of an important initiative by the International Surgical Wound Complications Advisory Panel (ISWCAP) to study surgical site infections on a global scale and highlight the need for more objective diagnostic techniques to support the early and accurate detection of clinically concerning bacterial burden in surgical wounds. The authors note that this is the first study reporting the use of an advanced diagnostic device for the visualisation and diagnosis of bacterial burden in surgical wounds.
“MolecuLight fluorescence imaging technology allows clinicians to see into the wound. The point-of-care imaging device enables clinicians to detect and manage elevated levels of bacteria to inform our decision-making,” says Dr. Thomas Serena, the publication’s contributing author, Founder and Medical Director of The SerenaGroup®, and Vice President of ISWCAP. “Management of bacterial burden should always begin with wound hygiene strategies (e.g., cleansing, debridement), and only escalate to antibiotics when essential.”
|
References: |
|
1 Sandy Hodgetts, K. et al., Int Wound J. 2021;1–11 |
|
3Hatch MD et al. J Shoulder Elbow Surg. 2017;26(3):472-477 |
|
4 Badia JM, et al. J Hosp Infect. 2017;96(1):1-15 |
|
5 McLaws ML et al. J Hosp Infect. 2003;53(4):259-267 |
|
6 Sullivan E et al. Surg Infect (Larchmt). 2017;18(4):451-454 |
|
7 Ban KA et al. J Am Coll Surg. 2017;224(1):59-74 |
|
8 Berrios-Torres SI et al. JAMA Surg. 2017;152(8):784-791 |
|
9 Institute CPS. Canadian Surgical Site Infection Prevention Audit. 2016 |
|
10 Si D et al. BMC Infect Dis. 2014;14:318 |
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercially released devices, including the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, provide point-of-care handheld imaging devices for the global wound care market for the real-time detection of wounds containing elevated bacterial burden (when used with clinical signs and symptoms) and for digital wound measurement. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant, unmet needs including food safety, consumer cosmetics and other key industrial markets.