Nadia Al-Samarrie




Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES Natalie Bellini, DNP, FNP-BC, BC-ADM, CDCE
ANAHEIM, Calif., July 5, 2023 /PRNewswire/ — Histologics LLC attended “WOCNext” in Las Vegas last June 4-6th to exhibit its novel debridement and wound biopsy fabric-curettage devices including for the first time, the SoftBiopsy®+D device. Over 1M cases by clinicians used Kylon® devices for colposcopic biopsy and curettage in the USA, in nearly every clinical setting www.histologics.com. The hooked medical (Kylon®) fabric tipped brushes gently remove and can trap tissue for biopsy for histology or microbiology. The debridement may be performed at a lighter “hygiene” level, extending all the way to “excisional” surgical methods when the brush array converts to micro-curettes if the fabric is pressed firmly and wiped or twisted into tissue.
Kylon® Fabric Debridement and Biopsy : Video Simulation
The cause of some wounds not to heal includes biofilm, and the Kylon® fabric devices can be used to obtain true tangential biopsy samples from the debrided wound base for lab testing. This is commonly done with the SoftBiopsy® product, solely used for biopsy that is sent to a laboratory. Histologics recently released SoftBiopsy®+D, a more versatile and durable version for clinicians, that can sustain longer procedure time for wound debridement as well as biopsy.
We have previously demonstrated the value of our Soft K-Rette® device to debride and sample crevice wounds, and Soft K-Cot® deployed on the finger for “Compassionate Debridement at Your Fingertips®”.
The scope of practice of most physicians and nurse clinicians vary from basic wound care to the most advanced surgical procedures where necrotic wound tissue must be mechanically or surgically removed. Advanced and basic wound care providers include many specialties in health care and procedures occur in clinics, hospitals, homes, facilities and other settings. Some providers that shy away from regular debridement (necessary to heal wounds) due to the invasive nature of the scalpel and sharp curette would be willing to clean wounds using the gentle Kylon® devices.
Please visit our wound care website to request free samples at www.histologicwc.com, or contact Lily Ramos at histologicswc@gmail.com, Toll Free: 888-235-2275.
SOURCE Histologics LLC

Eyüp Murat Kanber • Harun Gulmez

(SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction Alessandro Del Cuore, Rosaria Maria Pipitone, Alessandra Casuccio, Marco Maria Mazzola, Maria Grazia Puleo, Gaetano Pacinella, Renata Riolo, Carlo Maida, Tiziana Di Chiara, Domenico Di Raimondo, Rossella Zito, Giulia Lupo, Luisa Agnello, Gabriele Di Maria, Marcello Ciaccio, Stefania Grimaudo & Antonino Tuttolomondo

agonists versus sulfonylureas and the risk of lower limb amputations: a nation-wide cohort study Nikki C. C. Werkman, Johanna H. M. Driessen, Coen D. A. Stehouwer, Peter Vestergaard, Nicolaas C. Schaper, Joop P. van den Bergh & Johannes T. H. Nielen
The Wound Masterclass Podcast brings you our fifth episode! This time the topic is ‘Wound Care in the Military Setting’ and we are joined by two guests to share their extensive experience in the area : Lt Col Professor Steven Jeffery and Dr Aliza Lee.

implants via photothermally-triggered nitric oxide and immunotherapy for enhanced osseointegration

Referral Hospital in West Java, Indonesia

Marc Trubin • Sarah E. Eichinger • Annette Abraham • Shivanjali Shankaran

KIM DELMONICO

Low-cost and versatile patch can be customised for different types and sizes of wounds, and provides early warning of adverse conditions to facilitate wound care and management

As the director of podiatric medical education and co-chief of the podiatry division, I lead certified residency and fellowship training in wound care along with our parent organization, Council on Podiatric Medical Education. We are a renowned healthcare organization specializing in podiatry and a broad range of primary care and specialty services …

by Caroline Fife, M.D. | Jun 22, 2023


Healthcare social media content from around the Web gathered by MedPage Today staff
HOUSTON, June 22, 2023 (GLOBE NEWSWIRE) — Nuo Therapeutics, Inc. (OTCQB: AURX) (“Nuo”), a commercial stage medical device company pioneering leading-edge biodynamic therapies by focusing on emerging opportunities in the evolving healthcare landscape, is pleased to announce that Wound Care Advantage (WCA), the nation’s leading wound care consulting firm has added the Aurix® System to its formulary. Founded in 2002, Wound Care Advantage (WCA) has established a large network of successful wound healing programs with partner hospitals. Through a strong commitment to quality care and innovation, WCA has built financially sustainable wound care programs that have saved limbs and lives of more than 40,000 patients suffering chronic wounds.
“Diabetic foot ulcers pose a significant risk to patients and can be challenging for wound care centers to treat from both clinical and financial perspectives,” commented Dave Hazard, Nuo’s Vice President of Sales. “With thousands of commercially available wound care products, it can be extremely difficult for wound care centers to identify products that are both reimbursed by Medicare, and more importantly, that actually heal patients. We are excited to partner with Wound Care Advantage’s team of experts who rigorously vet each product that is placed on the formulary.”
The Platelet Rich Plasma gel produced by the Aurix System is cleared by the FDA for treating chronic wounds with a simple one-minute spin. In a clinical study performed with the Centers for Medicare and Medicaid Services (CMS), the Aurix System demonstrated a higher healing rate and a significant time to heal advantage as compared to other advanced healing modalities.
About Nuo Therapeutics
Nuo Therapeutics, Inc. is a commercial stage medical device company pioneering leading-edge biodynamic therapies by focusing on emerging opportunities in the evolving healthcare landscape. The Company’s Aurix System is a biodynamic hematogel that harnesses a patient’s innate regenerative abilities for the management of a variety of wounds.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements. These forward-looking statements may include statements that are predictive in nature and depend upon or refer to future events or conditions, and may include words such as “believes,” “plans,” “anticipates,” “projects,” “estimates,” “expects,” “intends,” “strategy,” “future,” “opportunity,” “may,” “will,” “should,” “could,” “potential,” or similar expressions. You are cautioned not to unduly rely on forward-looking statements. Forward-looking statements are based on current expectations, assumptions, and information available to the Company’s management and are subject to known and unknown risks, uncertainties and other factors which may cause actual results to differ materially from the forward- looking statements. These risks, uncertainties, and factors are discussed under “Risk Factors” and elsewhere in the Company’s public filings with the U.S. Securities and Exchange Commission from time to time, including the Company’s annual report on Form 10-K, quarterly reports on Form 10-Q, and current reports on Form 8-K. You are advised to carefully consider these various risks, uncertainties, and other factors. The Company expressly disclaims any intent or obligation to update or revise publicly these forward-looking statements except as required by law.
Contact:
David Jorden
djorden@nuot.com

Pseudomonas aeruginosa forms two subpopulations to improve colonization success Division is controlled by a genetic switch that affects c-di-GMP levels Identification of this division mechanism can aid in treatment strategies
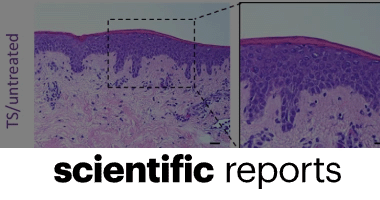
K. Parella, K. Moody, D. Wortel, H. Colegrove & J. A. Elser
Bay, Caroline C. BA; Grush, Andrew E. BS; Buchanan, Edward P. MD, FACS; Highfield, Linda PhD, MS; Krasnosky, Ryan MPAS, PA-C, DFAAPA, DrPH

Individuals with diabetes are at an increased risk of peripheral arterial disease (PAD), which can result in critical limb ischemia. Vascular surgery can prevent unnecessary limb amputations in individuals with critical limb ischemia. Written by Longjam Dineshwori
he Wound Masterclass Podcast brings you our fifth episode! This time the topic is ‘Wound Care in the Military Setting’ and we are joined by two guests to share their extensive experience in the area : Lt Col Professor Steven Jeffery and Dr Aliza Lee.

Shakeel Ahmad Khan, Adnan Shakoor

Wirawan Adikusuma, Zainul Amiruddin Zakaria, Lalu Muhammad Irham, Baiq Leny Nopitasari, Anna Pradiningsih, Firdayani Firdayani, Abdi Wira Septama & Rockie Chong


Without Pulmonary Involvement: A First-of-Its-Type Report Sankalp Yadav • Gautam Rawal • Madhan Jeyaraman

Morgan McCoy and Caroline Fife, MD, discuss Morgan’s wound care experience after a lower extremity amputation, and what physicians need to know about providing the best experience for their patients.
Former Healogics CEO Brings Deep Wound Care Industry Experience to MolecuLight’s
Rapidly Growing Business
TORONTO, July 6, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated, pathogenic bacterial loads in and around wounds, is pleased to announce the appointment of David Bassin to its Board of Directors as an Independent Board Member.
David Bassin brings a wealth of expertise and experience in the healthcare industry, particularly in the field of wound care. As the founder of GIO Advisory LLC, he has provided invaluable advisory services to numerous companies and private equity firms based on his extensive background in healthcare, spanning pharmaceutical/device, payer, and provider services. Most recently, Bassin served as the CEO of Healogics, the foremost provider of wound care services in the US, operating over 630 wound care centers and 300 providers. During his tenure, he successfully restructured and refocused the business, resulting in strong earnings growth prior to his transition into advisory services. Prior to his role as CEO, Bassin served as the CFO of Healogics, contributing to the company’s financial growth and success. Bassin’s impressive career also includes significant roles in other healthcare organizations. He served as the CFO of eviCore Healthcare, Inc., where he oversaw multiple dividend recapitalizations and facilitated a merger with the company’s largest competitor, leading to the creation of a company with over 3,000 employees. Additionally, Bassin held the position of CFO at InVentiv Health, Inc., a provider of services to pharmaceutical companies, where he successfully managed a high-growth company and orchestrated a go-private transaction valued at over $1 billion and delivered a significant premium to its shareholders.
“We are thrilled to welcome David Bassin to the MolecuLight Board of Directors,” said Anil Amlani, CEO of MolecuLight. “His extensive experience in optimizing and scaling organizations across the healthcare industry, particularly in wound care, will be invaluable to MolecuLight as we continue to expand our global presence. Our MolecuLight devices have already become indispensable tools in wound assessment and real-time decision-making for thousands of clinicians worldwide. David’s expertise will greatly support our mission to meet the increasing global demand for our innovative wound care diagnostics and establish them as the gold standard in the field.”
David Bassin expressed his excitement about joining MolecuLight’s Board of Directors, stating, “There are over 6.5 million patients living with wounds. As an industry, we need to continue to develop new solutions that improve wound care treatment effectiveness and efficiency. With a global drive to improve outcomes, reduce costs, and minimize antibiotic usage, MolecuLight’s point-of-care devices have demonstrated their ability to effectively address these critical clinical needs. I am deeply impressed by the organization, the technology’s alignment with market demands, and the significant market traction they have achieved. I am eager to contribute to their growth and help them achieve their ambitious goals.”
MolecuLight’s groundbreaking i:X® and DX™ imaging devices are the only FDA-cleared and CE and Health Canada approved devices for the real-time detection of elevated bacterial burden in wounds. Supported by over 80 peer-reviewed publications involving 2,600 patients, these devices are widely utilized by leading wound care facilities worldwide.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
For more information, contact:
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
T. +1.647.362.4684
rsandler@moleculight.com
www.moleculight.com
Image:
SOURCE MolecuLight


Several peptides have stood out from the rest for their potential benefits in wound healing, tissue repair, and regeneration. These best healing peptides include BPC-157, Thymosin Beta-4/TB500, Melanotan 2 (II), Sermorelin, and GHK-Cu

by Satish Sharma,James Mohler, Supriya D. Mahajan,Stanley A. Schwartz, Liana Bruggemann, Ravikumar Aalinkeel


GEORGIA INSTITUTE OF TECHNOLOGY
