UNIVERSITY OF BIRMINGHAM


UNIVERSITY OF BIRMINGHAM
The Wound Company, a multi-channel on-demand wound and ostomy care delivery company that improves patient outcomes, today announced its launch from stealth with $4.25M in seed funding from Susa Ventures and Sozo Ventures. The funding will be used to expand the company’s national footprint, hire top talent, and to continue improving health outcomes in the most cost-effective way possible while bringing dignity to the over 13 million people in need of improved wound and ostomy care.
Why Wound Care Matters
The US is experiencing an amputation epidemic due to diabetic foot ulcers and other serious wounds. Despite medical advancements, Americans are amputating double the number of limbs today than during the Civil War. About 50% of lower extremity amputations would have been preventable if patients with type 2 diabetes and foot ulcers had access to better healthcare. This issue is one of the problems The Wound Company is solving for.
Often providers need more wound care expertise or, due to understaffing, don’t have time to offer comprehensive care continuously, leaving patients to figure it out on their own. This leads to wound care patients returning to the hospital due to improper wound care. But it can be prevented.
“The wound and ostomy care industry is broken,” said Nima Ahmadi, founder and CEO of The Wound Company. “It’s operating in the fee-for-service world, which pushes expensive procedures and products that help the bottom line, but don’t impact outcomes for the patient. We’re paid to heal wounds with continuous care and, in doing so, save money for health plans and at-risk providers.”
Enter The Wound Company
The Wound Company aims to fix the broken space of wound and ostomy care by using predictive analytics and multi-channel communications to deliver the right wound and ostomy care to the right patient in the right channel at the right time. The tech connects patients and providers to wound and ostomy experts virtually or via in-person visits to ensure they have top-tier care.
“The Wound Company’s innovative technologies have the potential to save health plans billions of dollars and transform the patient experience,” says Susa Ventures Investor Derick En’Wezoh. “With a dedicated team of highly experienced experts, a strong vision, and a passion for improving healthcare outcomes, this tech will save lives.”
The platform also offers clinical reporting, customer data integration, and workflow automation to make care delivery as painless as possible for providers.
While in stealth, The Wound Company has already partnered with health plans, health systems, home care providers, hospice providers, and patients, with significant results to date:
“Our blend of virtual and in-person services provided by passionate experts in wound care helps people heal quickly, safely, and with the dignity they deserve while helping to alleviate the pressure on overworked healthcare professionals,” said Chief Medical Officer Sanford Roberts.
The Wound Company is open to partnerships with health plans, at-risk providers, home health providers, and hospice care providers. For more information, visit www.thewound.co.
About The Wound Company
The Wound Company is a Minneapolis-based technology company dedicated to advancing wound and ostomy care. The company uses proprietary technology to connect providers with experienced and certified wound care specialists who can care for patients virtually or via in-person visits. The Wound Company partners with health plans, home care companies, and providers to bring dignity to patients with wounds and ostomies while increasing positive patient outcomes.
This article was originally published here

Kathleen D. Schaum, MS

Hilliard T. Brydges BS, Grace McDonnell, Hani Y. Nasr MD, Bachar F. Chaya MD, Ogechukwu C. Onuh BA, Allyson R. Alfonso MD, Daniel J. Ceradini MD

Fayin Mo, Minjun Zhang, Xuewei Duan, Chuyan Lin, Duanping Sun, Tianhui You

pathophysiologic factors: A systematic review and meta-analysis Beshoy Girgis PT, MSc, Davide Carvalho MD, PhD, José Alberto Duarte MD, PhD

Kelsey McKee • Joseph Easton • Brian Mullis • Ivan Hadad

Xinyi Lin MD, Yan Wu PhD, Hong Huang MD, Ruihan Peng, FuHua Huang MD, Lvrong Hong MD, WenZhuan Chen MD

Discharge Program for Patients Receiving Negative Pressure Wound Therapy Leila Boti Laura Soloway Deb Myers Dinu Pillai Javad Zabihi
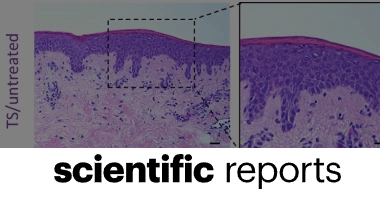
Mohamed Salah, Gamal Badr, Helal F. Hetta, Walaa A. Khalifa & Ahmed A. Shoreit
David G. Armstrong, DPM, PhD, is an internationally recognized leader in the field of podiatric surgery, diabetic foot, limb preservation, and wound healing.
DALLAS, TX, USA, June 12, 2023/EINPresswire.com/ — Venturis Therapeutics, Inc. (“VT” or the “Company”) announces that David G Armstrong, DPM MD PhD will assume the role of senior academic advisor for the surgeon board. This agreement was consummated following a review of the Company’s scientific and commercial opportunities.
Armstrong is the foremost expert in diabetic limb preservation and is recognized worldwide with a huge following through his prodigious authorship and lectures on diabetic limb salvage. This affiliation will further enhance the diabetic wound program and clinical research trials being undertaken by VT, and will improve the visibility of the Company dramatically among all those in the greater biotech wound healing community.
ABOUT VENTURIS THERAPEUTICS
Venturis Therapeutics, Inc. is a biopharmaceutical company developing protein drug candidates to address diseases such as severe coronary heart disease, diabetic wounds, peripheral artery disease, erectile dysfunction, stroke, and spinal disk disease. The active pharmaceutical ingredient (“API”) in our drug candidates is FGF-1, a human protein that stimulates the growth of new blood vessels, thereby increasing the blood supply to ischemic organs and tissues.
FORWARD LOOKING STATEMENTS
This news release contains forward-looking statements that involve risks and uncertainties. Actual results and outcomes may differ materially from those discussed or anticipated. For example, statements regarding expectations for new research, progress with clinical trials or future business initiatives are forward looking statements. Factors that might affect actual outcomes include, but are not limited to, FDA approval of VT drug candidates, market acceptance of VT products by customers, new developments in the industry, future revenues, future expenses, future margins, cash usage, and financial performance. Additionally, until VT is cash flow positive from operations, the Company is dependent upon raising capital to fund its operations and meet its obligations as they come due. There can be no assurance that VT will be able to raise the necessary capital when needed.
Amy Gordon
Venturis Therapeutics Inc
+1 972-904-2029
email us here
Dr. David G Armstrong The 18th Malvern Diabetic Foot Conference Future of Wound Treatment

by Caroline Fife, M.D.


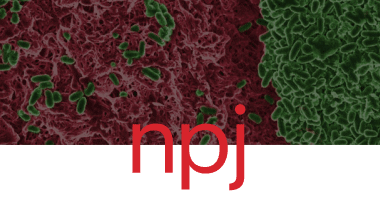
Ramón Garcia Maset, Alexia Hapeshi, John Lapage, Niamh Harrington, Jenny Littler, Sébastien Perrier & Freya Harrison

Kui-Xiang Wang,1 Li-Li Zhao,1 Ling-Tao Zheng,2 Li-Bin Meng,1 Liang Jin,3 Long-Jun Zhang,4 Fan-Lei Kong,1 Fang Liang
This polymer-based dressing might also be utilized to deliver chemotherapy in cancer treatment. Mrigakshi Dixit
RALEIGH, N.C.–(BUSINESS WIRE)–In the United States alone, a leg is amputated every two minutes. As announced at last week’s New Cardiovascular Horizons (NCVH) Annual Conference, this confronting statistic is being met head on by a digital innovation initiative referred to as SL2 for “Saving Limbs. Savings Lives.” Enrollment in SL2 is now open to all members of the American Podiatric Medical Association (APMA), the country’s largest non-profit organization dedicated to advancing the practice of foot and ankle medicine.
APMA members will have access to CarePICS, a software application purpose built to support best practices in wound care, including electronic consults and electronic referrals for optimal collaboration with peers in the vascular community. As an added benefit, many of the activities facilitated within CarePICS are eligible for reimbursement. The application may be used via mobile phones, desktop computers and iPads/tablets. It does not require any special devices.
For more information on SL2 and to enroll, visit https://carepics.com/apma/sl2.
The nationwide launch of SL2 is considered Phase 2 of the digital innovation initiative, with Phase 1 having drawn to a close at the end of May 2023, following a successful six-month pilot in Florida and Texas. Phase 2 will run for 12 months, through June 2023, at which time the potential for a permanent program will be evaluated.
The steadily increasing rate of lower extremity amputations in the United States – an estimated 60% deemed preventable – is shown to be the result of four key variables: (1) imprecise wound assessment and measurement (using manual tools), (2) inadequate and inconsistent wound documentation, (3) suboptimal patient follow-up and communication, and (4) fragmented coordination between providers who encounter wounds, such as podiatrists, and vascular specialists who treat associated medical conditions, mainly peripheral arterial disease (PAD) and critical limb ischemia (CLI). SL2 is positioned to combat these variables through a combination of digital tools, educational courses, data analysis and member support.
“When we look broadly at the medical histories of patients who have undergone lower extremity amputations, the evidence reveals that only about half have ever had a vascular evaluation or were referred to a vascular specialist. Their condition simply progressed to a stage where the limb could not be salvaged,” says Dr. Timothy Yates of Palm Vascular Centers, who participated in Phase 1 of SL2. “This is exactly the scenario SL2 is helping avoid. Using the CarePICS app, podiatrists can quickly and easily request an electronic consult with a vascular specialist, then convert it to an electronic referral when it’s indicated that the patient needs a vascular evaluation.”
“The CarePICS app has been a game changer for our wound care practice,” says Dr. Eric Lullove of West Boca Center for Wound Care, who also participated in Phase 1 of SL2. “Not only can we achieve precision and efficiency in our wound measurement and documentation, our patients can message with us, they can upload images, even participate in televisits. It is also possible to order wound dressings and cellular tissue products through the app.”
SL2 is governed by an advisory panel of physicians with expertise in podiatric and vascular medicine (in alphabetical order):
The SL2 advisory panel is directed by Christopher K. Bromley, DPM, FACFAS, Chief Medical Officer of CarePICS and Adjunct Professor at Kent State University College of Podiatric Medicine.
About CarePICS
CarePICS is a health tech company on a mission to save limbs and save lives through efficient, high-value digital tools purpose built to foster best practices in wound care, including precision measurement, compliant documentation, patient self-reporting, and streamlined collaboration among all providers in the continuum. CarePICS may be used in podiatry, vascular medicine, primary care, endocrinology, cardiology, oncology, plastic surgery, dermatology, geriatrics and myriad other clinical disciplines. The software platform serves as the backbone of two nationwide programs aimed at reducing preventable lower extremity amputations: SL2 and Collaborate4Wounds. CarePICS was founded by Paul Schubert and Terry Williams, both industry veterans of wound care and healthcare technology innovation. For more information, visit www.carepics.com.
Joy Efron, Principal
Kibit Marketing
joy@kibitmarketing.com
NEXA NPWT System Launched in the USA
OCEANSIDE, Calif., June 7, 2023 /PRNewswire/ — AOTI Inc, the global leader in multi-modality topical wound oxygen, announced exciting news from ongoing WOCNext conference in Las Vegas, Nevada, where their game changing NEXA Negative Pressure Wound Therapy (NPWT) system made its official USA debut.
The unique NEXA NPWT system is an incredibly flexible platform that is simple to use, silent, portable and affordable. NEXA seamlessly combines the simplicity of disposable NPWT with the performance features of more traditional durable NPWT technology platforms.
Dr. Mike Griffiths, CEO and President of AOTI commented; “Releasing the innovative NEXA NPWT platform in the USA is a major milestone for the company that will allow clinicians, payers, and patients alike to experience much improved performance at significantly lower cost. With NEXA, we have reimagined how NPWT should function.
Its addition to our portfolio only further enhances our mission of helping all people with chronic conditions get back to living their lives to the fullest.”
AOTI’s unique NEXA NPWT and Topical Wound Oxygen (TWO2) therapy are unlike any other treatment approaches. NEXA provided unrivaled flexibility and performance in a portable NPWT system. TWO2 is the only device that provides a multimodality treatment, combining higher pressure oxygen delivery with non-contact cyclical compression and humidity, in a therapeutic applied by the patient at home. This patented approach has been demonstrated in numerous Randomized Controlled Trial (RCT) and Real World Evidence (RWE) studies to not only heal chronic wounds at a far higher rate, but perhaps more importantly, keep them closed longer term, thereby reducing unnecessary hospitalizations and amputations.1, 2
1 Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers; The TWO2 Study. Robert G. Frykberg et al, Diabetics Care 2020; 43:616-624. https://doi.org/10.2337/dc19-0476.
2 Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes; Jessica Izhakoff Yellin, et al; Advances in Wound Care 2022; http://doi.org/10.1089/wound.2021.0118
About AOTI
AOTI is a privately-owned company based in Oceanside, California USA and Galway, Ireland that provides innovative solutions to resolve severe and chronic wounds worldwide. Our mission is to help all people with chronic conditions get back to living their lives to the fullest. We do this by enhancing access to care, improving quality of life and advancing health equity.
Our products reduce healthcare costs and improve the quality of life for patients with these debilitating conditions. Our patented Topical Wound Oxygen (TWO2) at home therapy is clinically proven to deliver Sustained Wound Healing that reduces both Amputations and Hospitalizations, So Life Can Get Back to Normal.
For more information see: www.aotinc.net
Contact:
Dr. Mike Griffiths
CEO & President
360322@email4pr.com
(760) 672 1920
SOURCE AOTI Inc.

A Cross-Sectional Study Yohei Tanaka • Takaaki Ueno


Foot and wound chronic disease is characterized by persistent ulcers, sores, and infections that primarily affect the feet. it is a condition that causes pain, affects mobility issues, and even long-term disability if left untreated. On today’s health beat, we delve deeper into the challenges faced by individuals living with foot and wound disease.

Sawsen Nouira • Taïeb Ach • Foued Bellazreg • Asma Ben Abdelkrim

In this webcast, Dr. Vincent W. Li reviews the biology of placental based allografts and the components relevant to wound healing, regeneration, and tissue repair. From May 2, 2019
To learn more about how amnio allografts can benefit your patients with chronic wounds as well as reimbursement information schedule a meeting at support@AmnioSource.com or call (336) 223-5050

Yusuf Can Edek • Elif Çalışkan Güneş • Hale Nur Ertugay Aral • Esra Adışen • Ahmet Burhan Aksakal


Dasman Diabetes Institute (DDI), established by the Kuwait Foundation for the Advancement of Sciences, held recently its specialized three-day course on the prevention and management of diabetic foot complications, in collaboration with the Primary Health Care Department at the Ministry of Health who assisted in coordinating this course. The program was held at the Institute…
BOULDER, CO / ACCESSWIRE / June 8, 2023 / Sonoma Pharmaceuticals, Inc. (Nasdaq:SNOA), a global healthcare leader developing and producing patented Microcyn® technology based stabilized hypochlorous acid (HOCl) products for a wide range of applications, including wound care, dermatology, and eye, oral and nasal care, today announced a new application for intraoperative pulse lavage irrigation treatment, which can replace commonly used IV bags in a variety of surgical procedures.
Sonoma developed this new application of its wound care technology in response to an unmet need for a non-toxic irrigation solution that can prevent infection and improve healing time. The intraoperative pulse lavage container is designed to be used in combination with a pulse lavage irrigation device, or flush gun, for abdominal, laparoscopic, orthopedic, and periprosthetic procedures. This product replaces commonly used non-antimicrobial saline and aggressive rinsing solutions with safe and effective Microcyn® Technology. Microcyn® Technology assists in the reduction of microorganisms, is non-toxic, and has regenerative properties, making it critical in preventing infection and promoting wound healing. Sonoma’s pulse lavage container is also cost competitive with IV bags, the current standard of care.
Sonoma developed the intraoperative pulse lavage irrigation treatment in close collaboration with the medical community and Sonoma’s existing distribution partners in Europe and expects this new application will be met with wide acceptance. Sonoma is now accepting orders for the pulse lavage irrigation treatment solution, which is expected to be ready for commercial use in Europe in September 2023. Sonoma anticipates commercial launch in the U.S. in 2024.
“Sonoma continues to lead in the innovation of products that improve outcomes for people with wounds or injuries or who are needing surgery. We continue to see increased demand for our wound care products in Europe, and we are excited to expand our offerings to include this next generation irrigation solution to help people heal faster following surgery,” said Amy Trombly, CEO of Sonoma Pharmaceuticals.
For more information, or to pre-order our pulse lavage irrigation treatment solution in Europe, please contact info.europe@sonomapharma.com.
About Sonoma Pharmaceuticals, Inc.
Sonoma Pharmaceuticals is a global healthcare leader for developing and producing stabilized hypochlorous acid (HOCl) products for a wide range of applications, including wound care, eye care, nasal care, oral care, dermatological conditions, animal health care and non-toxic disinfectants. The company’s products reduce infections, itch, pain, scarring and harmful inflammatory responses in a safe and effective manner. In-vitro and clinical studies of hypochlorous acid (HOCl) show it to have impressive antipruritic, antimicrobial, antiviral and anti-inflammatory properties. Sonoma’s stabilized HOCl immediately relieves itch and pain, kills pathogens and breaks down biofilm, does not sting or irritate skin, and oxygenates the cells in the area treated, assisting the body in its natural healing process. The company’s products are sold either directly or via partners in 55 countries worldwide and the company actively seeks new distribution partners. The company’s principal office is in Boulder, Colorado, with manufacturing operations in Guadalajara, Mexico. European marketing and sales are headquartered in Roermond, Netherlands. More information can be found at www.sonomapharma.com. For partnership opportunities, please contact busdev@sonomapharma.com.
Forward-Looking Statements
Except for historical information herein, matters set forth in this press release are forward-looking within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including statements about the commercial and technology progress and future financial performance of Sonoma Pharmaceuticals, Inc. and its subsidiaries (the “company”). These forward-looking statements are identified by the use of words such as “continue,” “develop,” “anticipate,” “expect” and “expand,” among others. Forward-looking statements in this press release are subject to certain risks and uncertainties inherent in the company’s business that could cause actual results to vary, including such risks that regulatory clinical and guideline developments may change, scientific data may not be sufficient to meet regulatory standards or receipt of required regulatory clearances or approvals, clinical results may not be replicated in actual patient settings, protection offered by the company’s patents and patent applications may be challenged, invalidated or circumvented by its competitors, the available market for the company’s products will not be as large as expected, the company’s products will not be able to penetrate one or more targeted markets, revenues will not be sufficient to meet the company’s cash needs, fund further development, as well as uncertainties relative to the COVID-19 pandemic and economic development, varying product formulations and a multitude of diverse regulatory and marketing requirements in different countries and municipalities, and other risks detailed from time to time in the company’s filings with the Securities and Exchange Commission. The company disclaims any obligation to update these forward-looking statements, except as required by law.
Sonoma Pharmaceuticals™ and Microcyn® are trademarks or registered trademarks of Sonoma Pharmaceuticals, Inc. All other trademarks and service marks are the property of their respective owners.
Media and Investor Contact:
Sonoma Pharmaceuticals, Inc.
SOURCE: Sonoma Pharmaceuticals, Inc.

The “Saving Limbs, Saving Lives” campaign aims to give healthcare providers access to a digital health platform that offers care management resources and a virtual connection to specialists
Come and learn all about debridement from our global wound care experts!

A new generation of wirelessly-powered, environmentally-friendly ‘smart bandages’ could help patients with non-healing wounds avoid infections, scientists say. The bandage could help improve the quality of life of people who live with chronic non-healing wounds, which currently frequently require painful cleaning and treatment. Non-healing wounds can be a side effect of certain medications or health factors like diabetes, cancer or damaged blood vessels. The smart bandage is built on technology initially developed by Dr Mahmoud Wagih of the James Watt School of Engineering and his colleagues from the University of Southampton. The research is showcased in a second paper, recently published in IEEE Transactions on Industrial Electronics. The paper demonstrates the first use of magnetic-resonant wireless power transfer to provide electricity to standard textiles using embroidery or screen printing – a feature which helped to make the smart bandage possible. In this case, the power was supplied to a newly-developed flexible electronic resistor made from silver and carbon which was printed into a textile surface to act as a wearable heating element. The system was capable of being heated to up to 60◦C while separated from the transmitter by 2cm at an efficiency exceeding 50%.
Swift’s AI-powered wound imaging technology shown to improve quality of care and costs to manage complex patients
June 01, 2023 09:00 AM Eastern Daylight Time
TORONTO–(BUSINESS WIRE)–Swift Medical, a digital health technology company focused on improving clinical and economic outcomes in wound care, today announced proven outcomes from more than 20 million wound assessments captured with Swift’s leading, AI-powered wound care platform. Deployed in nearly 4,000 healthcare facilities across North America, Swift’s technology has been shown to speed up wound healing by 37%, reduce wound prevalence and hospitalizations by 35% and 14%, respectively, and reduce hospital length of stay by 62%.
“We are proud to share the incredible impact our technology is having on the millions of patients living with wounds – one of today’s most expensive and overlooked threats to patients and our overall healthcare system”
Tweet this
“We are proud to share the incredible impact our technology is having on the millions of patients living with wounds – one of today’s most expensive and overlooked threats to patients and our overall healthcare system,” said Brian Litten, CEO of Swift Medical. “These outcomes demonstrate the impact of having the most powerful wound image dataset in the world and its ability to deliver high quality wound care with reduced costs.”
The Swift Skin & Wound mobile application enables any mobile device to be equipped with AI-powered imaging capabilities to capture clinically validated, high-precision 3D images, measurements, and other clinical data. The imaging captured at the point of care enables real-time, at-risk patient monitoring, proactive decision-making, and remote wound consultations, reducing the time and cost spent evaluating wounds to create a more efficient wound care experience for both clinicians and patients.
Today, more people suffer from chronic wounds than those with breast cancer, colon cancer, lung cancer, and leukemia combined. Despite this costly, growing problem, the current standard of care is outdated and highly inaccurate. Clinicians typically receive less than 10 hours of formal education and rely on paper rulers for measurements and cotton swabs for depth assessment. This inefficient and ineffective approach is both painful for patients and leads to poor diagnostic accuracy, prolonged healing, and inappropriate selection of therapies, putting patients at greater risk for readmission, longer lengths of stay and higher care costs.
With its advanced analytics and proven outcomes, Swift is now poised to partner with health plans and risk-bearing providers to deliver high quality, value-based wound care at scale.
About Swift Medical
Swift Medical is the global leader in digital wound care. We are headquartered in Toronto, with operations expanding across the U.S. and Canada. Our mission is to make empathy-driven wound care ubiquitous through AI-powered diagnostic technology. We are the trusted wound technology partner of more than 4,000 healthcare facilities in North America across the continuum of care. Our solutions empower healthcare providers to deliver standardized, accessible and equitable wound care for every patient – with advanced, high-precision imaging, compliant documentation, clinical analytics and remote care. To learn more about Swift Medical, visit us at www.swiftmedical.com.
Contacts
Media
David Mannion
416-303-8020
david.mannion@swiftmedical.com

Windy Cole, DPM, CWSP, FACCWS

of International Travel: A Case Report Andy Aleman Espino • Erik Aleman Espino • Claudia Aleman Oliva • Gustavo Huaman • Ricardo Castrellon
Comprehensive review of removing tissue from wounds for diagnostic or therapeutic (debridement/wound hygiene) with evidence and case examples. “Future of Debridement” segment by Dr. Neal Lonky and Dr. Traci Kimball ; May 31st, 2023 on Wound Masterclass


Michael King, Medical Director, Upperline Health

Study protocol for an international multicentre randomised trial Robin Brouwer, Rowan van der Peet, Rigo Hoencamp, Mark Koelemay, Susan van Dieren, Rob van Hulst, Dirk Ubbink


Joseph P. Kelly • Andrew S. Bae • Jacob Taunton • Achraf Jardaly • Robert M. Harris

Ieva Aleknaitė-Dambrauskienė, PhD


NANCY COLLINS

Nancy Collins, PhD, RDN, LD, NWCC, FAND

Brian McCurdy, the Managing Editor of Today’s Wound Clinic, and I talked about a project we are working on to elevate the patient’s voice in wound care Caroline Fife, M.D.


David Navazio


Virginie Papadopoulou, Ashelyn E. Sidders, Kuan-Yi Lu, Amanda Z. Velez, Phillip G. Durham, Duyen T. Bui, Michelle Angeles-Solano, Paul A. Dayton, Sarah E. Rowe

