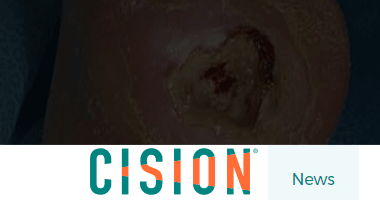
IR-Med’s PressureSafe Noninvasive Wound Care Device to be Demonstrated at Northwell Health’s 10th Annual Shining the Light on Wound Care Symposium


IR-Med’s PressureSafe Noninvasive Wound Care Device to be Demonstrated at Northwell Health’s 10th Annual Shining the Light on Wound Care Symposium

Robert Bem, Paul Chadwick, Ivan Cvjetko, Miroslav Koliba, Zoltan Kokeny, Przemyslaw Lipinski, Beata Mrozikiewicz-Rakowska, Istvan Rozsos, Adam Wegrzynowski
for the diagnosis and treatment of diabetic peripheral neuropathy Ye Yang & Qin Wang

Oxygen Therapy for Diabetic Foot Ulcers
Evidence-based recommendations support the effective use of TOT in diabetic foot ulcer management, revolutionizing treatment approaches worldwide
CAMBRIDGE, England, May 17, 2023 /PRNewswire/ — NATROX® Wound Care, a leading innovator in wound care technology, proudly announces the release of newly published expert recommendations on the use of topical oxygen therapy for wound healing1. The updated guidelines endorse topical oxygen therapy (TOT) as an adjunct therapy in the treatment of diabetic foot ulcers (DFUs) 1. With its endorsement by leading experts, this ground-breaking therapy is poised to transform the lives of millions of people worldwide, offering renewed hope for effective healing and improved quality of life.
New IWDGF Guidelines
The International Working Group on the Diabetic Foot (IWGDF) has just published its 2023 Guidelines. This set of recommended DFU interventions, developed by a panel of renowned experts, serves as a trusted resource for healthcare professionals worldwide.
Notably, among the 29 recommendations highlighted, TOT gained recognition as an accepted intervention when treating non-healing DFUs. “Consider the use of topical oxygen as an adjunct therapy to standard of care for wound healing in people with diabetes-related foot ulcers where standard of care alone has failed and resources exist to support this intervention1.” With its inclusion in the IWGDF guidelines, topical oxygen therapy emerges as a vital tool, poised to revolutionize the management and healing of foot ulcers in individuals with diabetes.
In addition, the guidelines note that “evidence on topical oxygen has substantially expanded in the last four years with several new RCTs with a total of ten included in the systematic review for these guidelines (References 100-109) 1“, which includes an RCT study published in 2021² which compared the healing effects of using standard care against a combination of standard care plus NATROX® O₂ topical oxygen therapy. In the study, patients completing the therapy experienced 71% greater healing rates² and 73% greater reduction in wound size² with NATROX® O₂.
Experts recommend updating algorithms to include TOT
In the Journal of Wound Care, experts reached a “clear consensus that adjunctive treatments with a solid evidence base, including NPWT and TOT, must be included“3 in each of the four proposed regional guidelines. Most notably, the experts agreed that “all hard-to-heal wounds are likely to benefit from TOT³.”
TOT received “A grade” from the American Diabetes Association
The American Diabetes Association recently released its “Standards of Care in Diabetes⁴” which not only recommended TOT as an adjunctive therapy for chronic DFUs, but also gave it an “A grade” based on the quality of evidence⁴. The newly published recommendations acknowledge the remarkable potential of TOT⁴.
According to Dr. Windy Cole, DPM, CWSP, FAPWH, FACCWS, renowned authority in podiatric medicine and dedicated wound care advocate for over two decades, “The evidence supporting the efficacy of TOT is now undeniable. It is imperative that healthcare professionals embrace this innovative yet simple approach to achieve improved healing outcomes.” After witnessing the positive impact topical oxygen therapy can have on healing DFUs in her own clinic, Windy recently joined the NATROX® team as Director of Global Medical Affairs to further advocate for the integration of topical oxygen therapy in the treatment pathway for chronic wounds.
NATROX® Wound Care CEO, Craig Kennedy, expressed great enthusiasm regarding the recognition and international acceptance of topical oxygen therapy, stating, “We’re delighted that topical oxygen therapy continues to gain international recognition, cementing its status as a game-changing treatment in wound care. The inclusion of topical oxygen in the IWGDF Guidelines further validates our mission to transform the quality of life for patients suffering from chronic wounds, particularly those with diabetic foot ulcers.”
What is NATROX® O₂ Topical Oxygen Therapy NATROX® Wound Care manufactures an award-winning5,6,7 topical oxygen therapy device known as NATROX® O₂. The compact, wearable device generates and delivers a continuous flow of oxygen directly to the wound bed to promote accelerated healing and foster a healthy wound environment. Its non-invasive nature, coupled with its remarkable effectiveness², offers a significant advancement in chronic wound treatment, even allowing patients to be treated from the comfort of home.
To learn about NATROX® O₂ and request a demo, visit: https://bit.ly/NO2therapy
About NATROX® Wound Care
NATROX® Wound Care is an Inotec AMD brand. The specialist wound care company based in Cambridge, England was formed specifically to introduce new technologies to healthcare professionals around the world to promote faster and better healing to patients. The company’s flagship product, NATROX® O₂, is positioned to become an integral part of global wound care treatment regimes in the coming years. To learn more, explore the website: natroxwoundcare.com.
See the references: https://bit.ly/nwc-iwgdf-guidelines
Media Contact:
NATROX® Wound Care
Nancy Stahulak
VP Global Marketing
marketing@natroxwoundcare.com
+1 (888) 354 9772
Noa Noach, Eran Lavy, Ram Reifen, Michael Friedman, David Kirmayer, Einat Zelinger, Amit Ritter, Dan Yaniv & Ella Reifen

Lei He, Huiying Lv, Yanan Wang, Feng Jiang, Qian Liu, Feiyang Zhang, Hua Wang, Hao Shen, Michael Otto & Min Li
Patients with diabetic foot ulcers randomized to receive an application of topical esmolol hydrochloride in addition to standard care were observed to have an improved rate of wound closure at 12 weeks

Researchers in the UNC School of Medicine’s Department of Microbiology and Immunology and the UNC-NC State Joint Department of Biomedical Engineering have developed a new strategy to improve drug-delivery into chronic wounds infections Sarah Rowe-Conlon, PhD Paul Dayton, PhD

Working Group on the Diabetic Foot in their 2023 Diabetic Foot Ulcer Guidelines OCEANSIDE, Calif., May 15, 2023 /PRNewswire/ — AOTI Inc, the global leader in multi-modality topical wound oxygen, announced exciting news from the 9th International Symposium on the Diabetic Foot (ISDF). The ISDF is often referred to as the Olympics of the Diabetic Foot, due…

by Kristen Monaco, Senior Staff Writer, MedPage Today May 15, 2023

Adjuvant Local Antibiotic Hydroxyapatite Bio-Composite Joshua A. Henry • Almigdad Ali • Ibrahim H. Elkhidir • Adam Reid • Jason Wong • Anand Pillai

led to Wound Treatment Plan Changes in up to 53% Cases Results from 211 Facilities Show MolecuLight Imaging is a Valuable Toolin Improving Bacterial Infection Management TORONTO, May 23, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated, pathogenic bacterial loads in and around wounds, announced the publication of their…

by Steve Bryson, PhD


Multi-center, randomized, placebo-controlled diabetic foot ulcer (DFU) Phase 2 study to start May 2023 in Italy, Germany, and Poland After completing 12 months follow-up of our DFU Phase 1 study, and 10M CHF Series A financing round to accelerate our clinical program, these are fantastic news for the company”— Juha Yrjänheikki, CEO BASEL, SWITZERLAND, May…
BOZEMAN, Mont., May 10, 2023 /PRNewswire/ – Microbion Corporation today announced that the company presented a poster focusing on pravibismane’s activity against diabetic foot ulcer infection pathogens at the 9th International Symposium on the Diabetic Foot that is currently ongoing from May 10th to 13th, 2023 at The Hague, Netherlands. The poster highlights pravibismane’s activity versus comparator antibiotics against pathogens isolated from diabetic foot infection (DFI) patients in an earlier Phase 1b clinical study. Poster Details:
Title: Broad-Spectrum, Potent Activity of Pravibismane Versus Comparators Against Diabetic Foot Ulcer Infection Patient Isolates Collected in a Phase 1b Study Presenter: Dr. Jeff Millard, CSO Poster Highlights:
"We are pleased that pravibismane demonstrated extremely potent MIC activity against clinical DFI isolates, which was in line with in vitro AST microbial pre-clinical studies," said Dr. Jeff Millard, CSO of Microbion Corp. "Diabetic foot infections are often infected by several different bacterial species concurrently, which may change over the chronicity of the wound, from predominantly aerobic to anaerobic. We believe pravibismane’s potent broad-spectrum activity is potentially a key treatment advantage since a single agent could eradicate both aerobic and anaerobic bacteria, thereby decreasing the need for multiple systemic therapies." Bacterial cultures for this study were grown from swabs collected at the wound bed at baseline visit and antimicrobial susceptibility testing (AST) was performed on isolated pathogens. Pathogen isolation and AST was performed at Investigational Health Management Associates (IHMA, IL), using the Clinical & Laboratory Standards Institute (CLSI) standard methods. Topical pravibismane has received QIDP and Fast Track drug designation from the US FDA for the adjunctive treatment of moderate and severe diabetic foot ulcer infections. Topical pravbismane is currently enrolling in a Phase 2 clinical study to further evaluate its safety and efficacy in subjects suffering from moderate infections associated with chronic diabetic foot ulcers. About Microbion
Microbion is a clinical-stage pharmaceutical company developing a new class of therapeutic compounds to improve the lives of patients with rare and serious diseases. Microbion’s lead drug candidate, pravibismane, is the first product in this new class and has multiple novel modes of action offering unique potential to address the unmet needs of chronic and severe health conditions. Topical/local pravibismane is in Phase 2 development for the treatment of chronic wounds and orthopedic infections. The Company is advancing inhaled pravibismane in Phase 1 clinical development for the treatment of chronic lung diseases, including non-tuberculous mycobacteria (NTM) and cystic fibrosis-related lung infections. Pravibismane has received backing from the Cystic Fibrosis Foundation, NIH, US DoD, and CARB-X with over $21 million in grants. The FDA has granted pravibismane with Orphan Drug, Fast Track, and QIDP designations. For more information visit: www.microbioncorp.com. Safe Harbor Statement
Certain of the statements made in this press release are forward-looking, such as those, among others, relating to the success of clinical development of pravibismane and preparation for potential commercialization. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: our ability to enroll patients in our clinical trials at the pace that we project; the size and growth of the potential markets for pravibismane or any future product candidates and our ability to serve those markets; our ability to obtain and maintain regulatory approval of pravibismane or any future product candidates; and our expectations regarding the potential safety, efficacy or clinical utility of pravibismane or any future product candidates. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Microbion Corporation disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. SOURCE Microbion Corporation |

The novel dressing reduces risk of complications and will contribute to the fight against antibiotic resistance.

Heather L. Barnhart, PT, PhD, CWS, AWCC, CLT-LANA, CLWT, CORE

by Caroline Fife, M.D.

Hendrik Lintel BS, Darren B. Abbas MD, Duncan J. Mackay MD, MBA, Michelle Griffin MBChB, PhD, Christopher V. Lavin MD, Charlotte E. Berry BS, Nicholas J. Guardino BS, Jason L. Guo PhD, Arash Momeni MD, Donald R. Mackay MD, DDS, Michael T. Longaker MD, MBA, Derrick C. Wan MD

Therapeutic Potential and Future Perspectives


Efficient Photodynamic Killing of Bacteria and Wound Healing Authors Hou B, Yang F, Hu C, Liu C, Xiao X, Chen Y, Huang X, Xie S

use in treatment of diabetic foot infection with or without osteomyelitis Gaielle Harb, Teri Hopkins, Linda Yang, Kathleen Morneau, Jose Cadena-Zuluaga, Elizabeth Walter & Christopher Frei

A meta-analysis Xinyi Lin MD, Yan Wu PhD, Hong Huang MD, Ruihan Peng, FuHua Huang MD, Lvrong Hong MD, WenZhuan Chen MD

Mohamed Salah, Gamal Badr, Helal F. Hetta, Walaa A. Khalifa & Ahmed A. Shoreit

Transition Discharge Program for Patients Receiving Negative Pressure Wound Therapy Leila Boti Laura Soloway Deb Myers Dinu Pillai Javad Zabihi

Kelsey McKee • Joseph Easton • Brian Mullis • Ivan Hadad

Saranya P. Wyles, Parisa Dashti, Tamar Pirtskhalava, Burak Tekin, Christina Inman, Lilian Sales Gomez, Anthony B. Lagnado, Larissa Prata, Diana Jurk, João F. Passos, Tamar Tchkonia, and James L. Kirkland from Mayo Clinic in Rochester, Minnesota

BOCA RATON, Fla., May 1, 2023 /PRNewswire/ — Hyperbaric Awareness USA™, a CutisCare initiative, has designated May Hyperbaric Awareness Month and is proud to announce the launch of the third annual Hyperbaric Aware™ national campaign to elevate awareness of hyperbaric oxygen therapy (HBOT). CutisCare launches a Hyperbaric Awareness USA campaign to elevate the awareness of hyperbaric oxygen…
Vincent Charron-Lamoureux, Lounès Haroune, Maude Pomerleau, Léo Hall, Frédéric Orban, Julie Leroux, Adrien Rizzi, Jean-Sébastien Bourassa, Nicolas Fontaine, Élodie V. d’Astous, Philippe Dauphin-Ducharme, Claude Y. Legault, Jean-Philippe Bellenger & Pascale B. Beauregard

Wound Management Association (EWMA) 2023 Annual Conference Wide-Spread Clinical Evidence using the MolecuLight Imaging Platform Reveals its Significant Global Adoption and Proven Utility in Wound Care TORONTO and MILAN, May 3, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that detects and locates elevated bacterial loads in wounds, announces that its MolecuLight wound imaging platform is featured in an unprecedented…




Subo Dey • Nirali Sanghavi • Amy Wasserman • Kausik Kar


Innovative therapy shown to help promote healing and reduce the need for surgical debridements creating potential savings for health care systems. ST. PAUL, Minn., April 27, 2023 /PRNewswire/ — 3M Health Care’s innovative 3M™ Veraflo™ Therapy, with both 3M™ Veraflo™ Cleanse Choice Complete™ Dressing and 3M™ V.A.C. Veraflo Cleanse Choice™ Dressing, received the first-ever Food and Drug Administration (FDA)…

by Caroline Fife, M.D.


By Natalie Sainz

Harvey N. Mayrovitz • Summer Wong • Camilla Mancuso

First Randomized Controlled Trial Specifically Powered to Assess Restoration of Neurological Function in Patients with Intractable Painful Diabetic Neuropathy Breakthrough Device Designation Provides Expedited Review for Marketing Application to Expand Nevro’s FDA Labeling REDWOOD CITY, Calif., April 24, 2023 /PRNewswire/ — Nevro Corp. (NYSE: NVRO), a global medical device company that is delivering comprehensive, life-changing solutions for the treatment…


— Should our practice add wound care and other offerings in a time of specialist shortages? by Fred Pelzman, MD, Contributing Writer, MedPage Today April 24, 2023

NEWS PROVIDED BY I3 BioMedical Inc. April 25, 2023, 11:00 GMT TrioMed Active Medical Technology that kills 99.9%+ of microbes in 5 minutes is now integrated in Disposable Slippers When you take your shoes off outside of your home, you expose yourself to foot infection, the Active Touch slipper provides you with the most advanced…

Detection of Bacterial Burden Across Patients of All Skin Tones Findings show that MolecuLight is an Objective and Equitable Diagnostic Technology Positioned to Help Level Racial Disparity in Wound Care Outcomes TORONTO, April 25, 2023 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging that locates and detects elevated bacterial loads in and around wounds,…

Researchers at Chalmers University of Technology in Sweden have developed a microfluidic system to test the effects of electrical stimulation in wound healing.


Hao Wang, J. Alex Chediak, Philip J. Belmont Jr, David M. Saylor & K. Scott Phillips

Increasing patient adherence to mechanical offloading will translate into better outcomes and a reduced number of amputations.
Olof Eskilson, Elisa Zattarin, Linn Berglund Kristiina Oksman Kristina Hanna Jonathan Rakar Petter Sivlér Mårten Skog Ivana Rinklake Rozalin Shamasha Zeljana Sotra Annika Starkenberg Magnus Odén Emanuel Wiman Hazem Khalaf Torbjörn Bengtsson Johan P.E. Junker Robert Selegård Emma M. Björk Daniel Aili
Yingnan Zhang, Khoi K. Do, Fuhua Wang, Xiaoqin Lu, John Y. Liu, Chi Li, Brian P. Ceresa, Lijun Zhang, Douglas C. Dean & Yongqing Liu


Liz Friedrich

Ulrich Joël Tsopmene • Yves Somo Iwewe • Isaac Mboh Eyong • Borel Ndezo Bisso • Jean Paul Dzoyem
Katharina Sommer, Heike Jakob, Theresa Lettenmeier, Dirk Henrich, Jasmina Sterz, Ingo Marzi & Johannes Frank

Northwell Health has been selected to join the Diabetic Foot Consortium as a satellite site and was awarded a grant by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Alisha Oropallo

by Caroline Fife, M.D.

the Caregivers of Diabetic Patients in Saudi Arabia Hassan Ali A. AlZubaidi • Ali Nori O. Alfaqih • Mohammed Hussain A. Alothayqi • Hassan Mohammed H. Alfaqih • Alaa Jameel A. Albarakati • Medhat Taha • Abdulkarim M. Alnashri


Sarah Sandmann, Jonathan Vas Nunes, Martin P. Grobusch, Maxwell Sesay, Martin A. Kriegel, Julian Varghese & Frieder Schaumburg

Autologous platelet-rich plasma (PRP) or autologous conditioned plasma (ACP) is widely employed in a variety of therapeutic applications. Here’s how it helps in treatment of chronic nonhealing ulcers.

by Caroline Fife, M.D.
Pieter T. Boonen , Dimitri Aerden


Bedsores with dead tissue either get infected leading to sepsis or even disseminated intravascular coagulation that leads to uncontrolled bleeding.

AlloPatch® Pliable Meshed is ideal for the treatment of diabetic foot ulcers and venous leg ulcers.
TISSUE ALLOGRAFTS FOR CHARCOT FOOT

Otomi O. Obuh • Ena-Jane O. Esomu • Roseline O. Sydney

Neha Chatterjee • Nishith M. Ekka • Mayank Mahajan • Binay Kumar • Nabu Kumar • Arquam Zia • Aravind Devarajan • Archana D. Kujur • Dipendra K. Sinha

Mark Hinkes DPM FACFAS FAPWCA DABFAS

Ze-Jun Pei, Chengcheng Li, Wenna Dai, Zaixiang Lou, Xin Sun, Hongxin Wang, Azmat Ali Khan, Chunpeng Wan
