Alisha Oropallo, MD, FACS, FSVS, FAPWCA, FABWMS


Alisha Oropallo, MD, FACS, FSVS, FAPWCA, FABWMS







and Ostomy® announces partnership to award wound care scholarships in Barbados




Dressing Demonstrates Bioabsorption and Biocompatability in Internal Bleeding


Readers who regularly frequent these pages are probably familiar with the topic of wound care.
One of the common causes of amputation of lower extremities


Tuesday October 11th, 12:00PM – 1:00PM EDT

BY PAUL KESSELMAN, DPM

By Dr. Lenz and Dr. Hussain

Amelia Beatty, DNP, MSN, NP, RN, CWCN-AP Penny S. Jones, AGNP-C, MN, CWS, CWCN-AP, COCN Christopher Vail, PA-C, MMCi Julie A. Thompson, PhD Staci S. Reynolds, PhD, RN, ACNS-BC, CCRN, CNRN, SCRN, CPHQ

Terry Treadwell

A Clinical Experience in Two Patients Nima Khavanin and Hooman T. Soltanian



Christine Miller, DPM, PhD

Laura Swoboda, DNP, APRN, FNP-C, FNP-BC, CWOCN-AP

by Caroline Fife, M.D.



Lower Extremity Wounds in Patients With Diabetes: A Case Series

Therapy System after Evaluation Yields Positive Results




Thursday, September 29, 2022 | 1:00 – 2:00pm ET

September 29 – October 1


from Jul 16, 2022

Nancy Collins, PhD, RDN, LD, NWCC, FAND

In this video, David Armstrong, PhD, DPM, discusses how DHACM treatment led to reductions in amputations, hospital readmissions, and cost for patient with diabetic ulcers.
Contract Awarded for Products that Bring Improvement to the Health Care Industry
PITTSBURGH, Sept. 29, 2022 /PRNewswire/ – MolecuLight Corp., the leader in point-of-care fluorescence imaging for the real-time detection of bacteria in wounds, announces it has been awarded a new group purchasing agreement with AllSpire Health GPO, a Mid-Atlantic GPO and a partner of HealthTrust Purchasing Group, engaged with over fifty hospitals in Maryland, New Jersey and Pennsylvania. AllSpire helps health systems optimize their operations by aggregating purchasing volumes, expenses, streamlining supplier negotiations and implementing efficiencies across the supply chain. The MolecuLight i:X® and DX™ wound imaging devices, which will now be available to AllSpire’s members, are helping clinicians to improve the state of wound care and ultimately to improve outcomes.
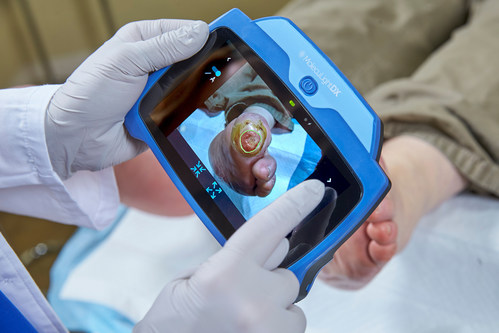
MolecuLightDX point-of-care imaging system for detection of elevated bacterial loads in wounds and for performing digital wound measurement and tracking (CNW Group/MolecuLight)
The MolecuLight imaging devices are the only FDA-approved devices that allow clinicians to visualize the presence, location, and load of bacteria (>104 CFU/g) in wounds in real-time. Published results from a recent 350-patient, 14-site clinical trial showed that the clinical standard of care alone detected 15% of wounds with elevated bacterial burden, while the addition of the MolecuLight device led to a 400% improvement in detecting these wounds2. The presence of elevated bacterial loads is known to impede wound healing1 and removal of bioburden is critical to improved wound outcomes1. The i:X and DX provide invaluable bacterial information at the point-of-care to inform clinical decision-making and enable targeted wound therapies. In a 2022 randomized controlled trial (RCT) 3, the highest level of evidence-based research, the improvement in healing rate at 12 weeks doubled in the patients that had care informed by MolecuLight fluorescence imaging compared to those that were not. Improvements in the patients’ quality of life were also reported. Another recent study reporting increased wound healing rates with the incorporation of bacterial information from MolecuLight imaging also found substantially decreased use of antimicrobial dressings and systemic antibiotics4. The MolecuLight devices also perform accurate digital wound measurement, allowing for the consistent monitoring and documentation of wounds.
“We are thrilled to have entered into a supply contract with AllSpire Health GPO,” says Anil Amlani, MolecuLight’s CEO. “Through the i:X and DX, we hope to enable significant cost-savings and improvements in clinical outcomes. AllSpire’s extensive member base can now easily access the MolecuLight wound imaging devices and see the clinical benefits in their wound care practices.”
“We are most impressed with the clinical utility that the MolecuLight i:X and DX devices provide to wound care professionals and are pleased to offer the MolecuLight portfolio via our Group Purchasing Agreement to our member hospitals”, says James Wallick, Senior Director, Strategic Sourcing at AllSpire Health GPO. “AllSpire is dedicated to sourcing the most innovative products that help to improve clinical decision-making and cost-efficiencies. We believe that the MolecuLight devices are highly innovative and will help to provide such clinical and operational benefits”.
In addition to the clinical benefits, MolecuLight procedures performed in the United States can benefit from an available reimbursement pathway including two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” procedures and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment.
About MolecuLight Corp.
MolecuLight Corp. is the US subsidiary of MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercially released devices, including the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, provide point-of-care handheld imaging devices for the global wound care market for the real-time detection of wounds containing elevated bacterial burden (when used with clinical signs and symptoms) and for digital wound measurement. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant unmet needs including food safety, consumer cosmetics and other key industrial markets.
About AllSpire Health GPO
Founded in 2016, AllSpire Health GPO, LLC is a collaborative, regional group purchasing organization utilizing innovation analytics, as well as product and purchased service standardization, as a platform to escalate the improvement of clinical outcomes, enable greater access to affordable healthcare, ensure economic sustainability, and enhance patient, physician, and clinician satisfaction among its members. AllSpire Health GPO delivers value via the development and execution of clinical and service line improvement initiatives across the care continuum.
SOURCE MolecuLight



of chronic diabetic foot ulcers at the world’s premier diabetic foot conference (DFCon) in Los Angeles


Robyn Rayner, Keryln Carville, Joanna Smith and Cate Maguire

Jonathan Quinn, William Fairbairn, Gayle Silveira and Simon Platt


ALLOGRAFT APPLICATION IN ADVANCED VENOUS LEG UCLERS







Physician Fee Schedule: Changes Could Increase Amputations and Infection for Patients with Wounds, Restrict Access to Care, Impact Reimbursement



Jun Ho Park & Ji-Ung Park



Bacterial biofilms pose a therapeutic challenge to managing chronic wounds and contribute to antimicrobial resistance. Here, Powell et al. investigated the structure/activity relationships of epoxy-tigliane compounds derived from the blushwood tree with respect to their role in wound healing.

The biofilm community has historically been very successful in aggregating scientists from very diverse fields. Now, we must harness innovative technologies across disciplines to illuminate the biofilm microenvironment and create in vitro models that accurately recapitulate natural environments.

Standardized methodology demonstrates high presence of placental membrane growth factors in BioStem’sVendaje® and Vendaje AC®tissue allografts

Skeleton buried in Borneo cave suggests early artists were also early surgeons
CellResearch Corporation, a Singapore-based biopharmaceutical company today announced it has successfully closed the first Phase I study in CorLiCyte®, a stem cell therapy derived from umbilical cord lining stem cells, with research partners at the University of Colorado, Anschutz Medical Campus and ClinImmune Cell and Gene Therapy.


by Bari Weiss


Advocacy on Behalf of Members and Collaboration with Government Agencies




Guoxin Tan, Lijie Wang, Weisan Pan, Kai Chen

as First Aid Kits are Restocked During National Preparedness Month

CANTON, Mass., Sept. 07, 2022 (GLOBE NEWSWIRE) — Organogenesis Holdings Inc. (Nasdaq: ORGO), a leading regenerative medicine company focused on the development, manufacture, and commercialization of product solutions for the Advanced Wound Care and Surgical and Sports Medicine markets, today announced that company management will participate in the Morgan Stanley 20th Annual Global Healthcare Conference, which is being held at the Sheraton New York Times Square Hotel in New York, NY from September 12th-14th. Management will host a fireside chat on Tuesday, September 13th at 11:45 a.m. Eastern Time.
A live audio webcast of the conference presentation will be accessible by visiting the “Upcoming Events” section on the “Investor Relations” page of Organogenesis’s website www.organogenesis.com. An archive of the webcast will be available for replay following the conference for approximately 30 days.
About Organogenesis Holdings Inc.
Organogenesis Holdings Inc. is a leading regenerative medicine company offering a portfolio of bioactive and acellular biomaterials products in advanced wound care and surgical biologics, including orthopedics. Organogenesis’s comprehensive portfolio is designed to treat a variety of patients with repair and regenerative needs. For more information, visit www.organogenesis.com.




Lihong Chen, Yunyi Gao, Yan Li, Chun Wang, Dawei Chen, Yun Gao, Xingwu Ran
The regenerative biotechnology is reducing healing times to as little as three weeks in Mohs-related surgical wounds
PHOENIX–(BUSINESS WIRE)–Dermistat™, one of the latest wound healing innovations by BioLab Sciences, a regenerative biotechnology company, has emerged as the go-to wound healing option for dermatologists throughout the country. Introduced earlier this year, Dermistat™ is a revolutionary regenerative therapy that is transforming the wound care industry by decreasing healing time significantly compared to the standard of care secondary closure.
 Part of the MyOwn Skin™ suite of products, Dermistat™ uses a small sample of the patient’s healthy skin to create a gel-like substance that can create an autologous skin graft in as little as 48 hours. Used for acute surgical wounds, including Mohs procedures unable to be closed with standard plastic surgery techniques, and non-healing wounds, Dermistat™ is non-invasive, improves recovery time, is relatively painless, and creates only a small, superficial donor site wound.
Part of the MyOwn Skin™ suite of products, Dermistat™ uses a small sample of the patient’s healthy skin to create a gel-like substance that can create an autologous skin graft in as little as 48 hours. Used for acute surgical wounds, including Mohs procedures unable to be closed with standard plastic surgery techniques, and non-healing wounds, Dermistat™ is non-invasive, improves recovery time, is relatively painless, and creates only a small, superficial donor site wound.
“Dermistat™ was originally developed to treat severe burns, trauma and plastic procedures, however, BioLab Sciences modified the process enabling it to be effectively leveraged in the dermatologist’s office, allowing patients to recover faster and more completely,” said Jaime Leija, Chief Commercialization Officer at BioLab Sciences. “Dermistat™ is proving to be a valuable resource for Mohs patients as it eliminates the need to perform a graft to cover larger wounds and it significantly reduces the healing time.”
This novel biotechnology is based on the MyOwn Skin™ technology, which leverages a very small sample of a patient’s own skin through a non-surgical procedure to reproduce the gel-like substance within 48 hours. In many cases, Dermistat™ accelerates the healing of post-surgical and chronic wounds and because of its favorable outcomes, it is on track to disrupt the wound care industry.
“We have been using Dermistat™ on surgical defects post-Mohs surgery after excising skin cancer,” said Travis Gilbert, PA-C with Desert Dermatology & Skin Cancer Specialists. “The wounds were typically open for 5-7 days prior to applying Dermistat™. Prior to using Dermistat™, we would close with flaps mostly. Occasionally, we would use skin grafts. Today we are using mostly Dermistat™ to cover these acute surgical wounds. They are healing much more rapidly than secondary closure and do not require significant plastics closure.”
About BioLab Sciences
BioLab Sciences is a regenerative medicine company focused on creating new ways to heal the body. The company is uncovering better ways to address orthopedic injuries, wound care, pain management, aesthetic medicine, respiratory ailments, cardiovascular indications, ophthalmic issues, and more. Learn more at www.biolabsciences.net/.
Contacts
Beth Cochran, Wired PR
beth@wiredprgroup.com
(602) 758-0750

Pragateshnu Das, Debmalya Bhattacharya, Rajlaxmi Sathpathy
Mary R. Brennan MBA, RN, CWON

By Heidi Cross, MSN, RN, FNP-BC, CWON

Alton R. Johnson, Jr., DPM, FACPM, CWSP

Mandalyn Mills Luke Counterman Shanna Williams

Sara Yumeen Mélissa Roy Fatima N. Mirza Sarah Rehou Shahriar Shahrokhi


Asad Ullah, Syed I. Jawaid, Pir Naveed Ahmed Ahsan Qureshi, Tehreem Siddiqui, Khadija Nasim, Kantash Kumar, Shafqat Ullah, Mustafa Sajjad Cheema, Nikita Kumari, Hafiza Azad Elias
BEVERLY HILLS, Calif., Aug. 23, 2022 (GLOBE NEWSWIRE) — Miracle Dressing Wound Care System 21-Day Stay-in-Place Dressing has been named Wound Care Product of the Week by WoundSource, “the world’s definitive source for wound care and product information.”
Miracle Dressing™ Wound Care System is the only 21-day stay-in-place dressing. Wound prevention, monitoring, cleaning and topical applications can all be completed without dressing removal. This saves nursing time and reduces patient distress from frequent dressing changes.
The system includes Natural Marine Extract™, the ingredients of which are known to promote faster healing of the skin and better wound healing results.
The Wound Healing Society spotlighted the importance of dressings that can stay in place for an extended time, especially due to extended stays caused by COVID mitigation. They recommend utilizing dressings that decrease the intensity of wound care by avoiding the use of dressings that must be changed daily. This would allow over-stressed health care staff to reach more patients in an adequate time frame.
The Miracle Dressing System can be used for:
MBET is recognized for their skin revitalization and wound care products as well as kelp reforestation and other international environmental projects.
MBET Health is a solutions-oriented technology company focused on solving one of the most important and intransient problems facing the world of medicine: preventing and healing wounds.
MBET Health was founded by Dr. Eric Lewis, a practicing dermatologist, surgeon and scientific researcher based in Beverly Hills, CA. The MBET Health management team includes medical and surgical doctors from a multitude of disciplines, pharmacists and marine biologists.
Beginning in 2001, their founders researched a multitude of marine compounds reported to have human health benefits. As a result of their extensive studies and tests, several compounds and systems specifically designed for rejuvenation and effective repair of damaged skin have been patented (or patent-pending). The mechanisms of action of their system’s ingredients are designed to heal intractable wounds and strengthen weak, vulnerable skin to minimize the probability of breakdown.
The MBET Health website provides a destination for all wound care providers, nursing staff and senior management to see for themselves the convincing before and after photos and to learn details about proper product application and dressing maintenance.
Contact
Linda Sherman Gordon
MBET Health Chief Marketing Officer
310-243-6305
Email contact
MBET Health LinkedIn
Before / After press photos available upon request
This article was originally published here

Neal M. Lonky, MD, MPH, Jeffrey Lehrman, DPM, FASPS, MAPWCA, CPC, Andrew Schneider




via Regulating Angiogenesis, Inflammatory Response and Skin Flora


Briana Fernandez, Sidra Deen, Carly Dunn, Tara L. Braun


Holly D. Sparks, Serena Mandla, Katrina Vizely, Nicole Rosin, Milica Radisic & Jeff Biernaskie


Patrícia Rosinha, Miguel Saraiva, Lia Ferreira, Susana Garrido, André Carvalho, Cláudia Freitas, Cláudia Amaral, Luís Costa, Luís Loureiro, Rui Carvalho



Shivani J. Patel, BS Khurram Khan, DPM, FACFAS, FACPM James McGuire, PT, DPM, CPed, FAPWHc

Posted by Tim Gilmer in Life After Paralysis on August 12, 2022

MANCHESTER CENTRAL, 22ND SEPTEMBER 2022
LARGO, Fla.–(BUSINESS WIRE)–BioDerm, Inc., a leading provider of disposable medical devices and wound care supplies, announced the addition of two executives to strengthen its leadership position in providing premium solutions for patients with chronic medical conditions. BioDerm President & CEO Gaet Tyranski said, “I’m ecstatic to add seasoned talent in the areas of sales and marketing and compliance to take us to the next level.”
 Colleen Kennedy is joining BioDerm as Vice President of D2C Sales and Marketing, bringing more than 20 years’ experience driving D2C marketing programs. Most recently, Colleen directed agency teams at Bluewater Media, working with a variety of D2C brands to maximize revenue opportunities. She specialized in multi-channel D2C programs that combined both traditional and emerging media channels to generate positive results through data driven analysis. Colleen has the unique ability to talk strategy in lay terms to guide teams in new and profitable directions, while building relationships based on trust and confidence. Prior to Bluewater Media, Colleen was the VP Account Director at D2C agency Acquirgy.
Colleen Kennedy is joining BioDerm as Vice President of D2C Sales and Marketing, bringing more than 20 years’ experience driving D2C marketing programs. Most recently, Colleen directed agency teams at Bluewater Media, working with a variety of D2C brands to maximize revenue opportunities. She specialized in multi-channel D2C programs that combined both traditional and emerging media channels to generate positive results through data driven analysis. Colleen has the unique ability to talk strategy in lay terms to guide teams in new and profitable directions, while building relationships based on trust and confidence. Prior to Bluewater Media, Colleen was the VP Account Director at D2C agency Acquirgy.
BioDerm promoted Alicia Lance to Sr. Director of Compliance for BioDerm and all affiliates. Alicia started at BioDerm in 2013, as a Manager of AMDI and most recently served as the Director of Customer Care and AMDI. She has built a strong customer care program at BioDerm based on integrity and dedication to patient outcomes. Alicia serves on the Medical Supply committee for AAHomecare and has over 20 years’ experience working in DME operations and compliance. She is excited to direct and expand BioDerm’s compliance program to enhance the company’s growth. Prior to BioDerm, Alicia worked at CCS Medical as a Compliance Manager. She holds a BS in Allied Health from the University of West Alabama.
About BioDerm
Headquartered in Largo, Florida, BioDerm is a leading provider of disposable medical devices and wound care supplies to patients with chronic conditions. BioDerm manufactures proprietary hydrocolloid products for urinary management, securement, infection control and skin protection, challenging accepted inferior standards of care by creating products that reduce infection rates, add comfort and reliability, and vastly improve quality of life. BioDerm’s flagship products for male urinary incontinence are Men’s Liberty for the home setting and Men’s Liberty Acute for in-patient settings. Other products include CathGrip for securement and BioPlus and FreeDerm for skin protection.
Wound Care Resources (“WCR”), a subsidiary of BioDerm, provides infection control products and other wound management supplies to patients with ventricular assist devices (VADs) installed due to heart failure, as well as other conditions.
For more information on BioDerm’s line of products visit www.bioderminc.com.
Contacts
Amy Stephens
Director of Marketing
727-416-2684
View source version on businesswire.com: https://www.businesswire.com/news/home/20220817005217/en/

From 6/25/2019
Medical groups say untreated chronic wounds are costing the Australian health systems $3 billion a year. Subscribe: http://ab.co/1svxLVE
Around Australia there are 420,000 people living with chronic wounds, with those living outside the big cities, poorer people and First nations people the worst affected.
Hayley Ryan, chair of advocacy group Wounds Australia, speaks with ABC News.

Natalie Vaughn

Periwound skin, found around a wound, is fragile and prone to injury

David D Zabel, MD, FACS
It is not clear what methods Dr Yusuf Maraşlı uses to reduce amputations, this might be worth a watch anyway:
Wound Healing Society for immersive educational experience
Premier annual limb salvage meeting co-locates with the leading meeting dedicated to the research, management, treatment, and prevention of wounds in April.
Three industry-leading wound care organizations are uniting for a conference in April 2023, focusing on wound prevention and management, research, and limb salvage. The meeting will offer the most cost-effective, comprehensive, and immersive educational event for professionals in the wound care space.
The Symposium on Advanced Wound Care (SAWC) Spring | Wound Healing Society (WHS) is the leading meeting dedicated to the research, management, treatment, and prevention of wounds; and Diabetic Limb Salvage (DLS) is the premier annual limb salvage education event focused on wound healing and preventing amputations. The 2023 event is organized by HMP Global, the omnichannel leader in healthcare events and education, and will be held April 26-30, 2023, in National Harbor, Maryland.
The in-person event will provide opportunities for networking and collaboration, with an educational agenda featuring an expert lineup of faculty, in-depth discussions, and exposure to innovation, proven techniques, and effective strategies for patient care. The interdisciplinary agenda is designed for every clinician interested in wound care, including physicians, nursing professionals, physical therapists, researchers, scientists, podiatrists, and dietitians — connecting the entire wound care team with the foremost experts in the field to improve patient outcomes through education.
DLS Co-Chairs are Dr. Christopher E. Attinger, Chief, Division of Wound Healing at MedStar Georgetown University Hospital and professor of plastic and orthopaedic surgery, Georgetown University School of Medicine in Washington, D.C.; and Dr. John S. Steinberg, Co-Director, MedStar Health Wound Healing Institute at MedStar Georgetown University Hospital, Hospital Center Director of the Podiatric Residency Training Program, MedStar Health, and a professor of plastic surgery at Georgetown University School of Medicine.
“Through this collaboration, educational opportunities at the conference will focus on every aspect of wound research, prevention, and healing along with a focus on limb salvage,” said Dr. Steinberg. “We are solving the issue of access to education by uniting these three events this year to provide learners with one comprehensive event. It is an opportunity for providers to strengthen their clinical skills, invigorate their approach, and positively impact their ability to care for their patients.”
The collaboration with DLS will add more limb salvage-focused topics to the conference agenda, including:
The WHS President is Dr. Kenneth Liechty, Division Chief of Pediatric Surgery and Director of Fetal Medicine, University of Arizona, and Surgeon in Chief of Diamond Children’s Hospital; and Co-Chairs are Dr. Daria Narmoneva, associate professor, University of Cincinnati, and Dr. Carlos Zgheib, assistant professor of surgery, University of Colorado Denver School of Medicine in Aurora.
“Although each of these three organizations have a unique mission, we are united in our goals of improving outcomes for patients and patient populations,” Dr. Liechty said. “We are excited to host one symposium for every member of the wound care team, allowing us to provide the highest caliber training and education that all clinicians can incorporate in their practice.”
SAWC Spring Co-Chairs are Dr. Robert S. Kirsner, Harvey Blank Professor and chairman, Dr. Philip Frost Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine; and Dot Weir, RN, CWON, CWS, clinician at Saratoga Hospital Wound Healing.
“We have partnered with the Wound Healing Society for 15 years, providing a robust educational experience for meeting participants, and this year’s event will be even stronger with the addition of multiple topics on amputation prevention,” Dr. Kirsner said. “No other wound care conference offers the level of education, advanced state-of-the-art clinical reviews, and emerging research findings.”
For more information or to register, visit sawcspring.com.
ABOUT HMP GLOBAL
HMP Global is the force behind Healthcare Made Practical — and is an omnichannel leader in healthcare content, events, and education, with a mission to improve patient care. The company produces accredited medical education events — in person and online via its proprietary VRTX virtual platform — and clinically relevant, evidence-based content for the global healthcare community across a range of therapeutic areas. Its brands include the HMP Global Learning Network, healthcare’s most comprehensive source for news and information; Psych Congress, the largest independent mental health meeting in the U.S.; the Evolution of Psychotherapy, the world’s largest independent educational event for mental health professionals; the Leipzig Interventional Course (LINC), the leading, global gathering for interdisciplinary cardiovascular specialists; EMS World Expo, North America’s largest EMT and paramedic event; and the Symposium on Advanced Wound Care (SAWC), the largest wound care meeting in the world. For more information, visit hmpglobal.com.

Alex K. Wong MD, FACS,

Jason Anderson, DPM, FACFAS

Charles B Parks, DPM, FACFAS
This includes the analogous nature of articular cartilage, muscle fascia, and intervertebral disc confirmed by way of comparative Scanning Electron Microscope analysis
PENSACOLA, Fla., Aug. 15, 2022 /PRNewswire/ — Regenative Labs, a leading HCT/P manufacturer, has co-authored a pioneering paper together with experts from The Institute of Regenative Medicine and the Department of Pharmacology and Chemical Biology, Baylor College of Medicine.
“This paper is a market disruptor and will be our most significant paper released to date. This is the first literature that we are aware of to utilize Scanning Electron Microscope (SEM) images of actual tissue samples to objectively demonstrate on a qualitative and quantitative basis that collagen structural tissue matrices in our post-processed Wharton’s Jelly allografts and those in articular cartilage, muscle fascia, and intervertebral discs are analogous,” said Regenative Labs CEO, Tyler Barrett.
This research highlights our commitment to the Regenerative Medicine community. We believe the combination of our IRB-approved observational studies, peer-reviewed publications, ISO-certified laboratory processes, and our commitment to compliance with FDA and American Association of Tissue Banks (AATB) standards, sets the standard for HCT/P manufacturers. Regenative Labs has pioneered the use of perinatal tissue allografts and is pleased that this paper supports our current homologous use practices, consistent with our 361 status.
Currently, the treatments for the Intervertebral Disc (DDD) range in cost and effectiveness from an $8 bottle of Ibuprofen to $150,000 for spinal fusion (1). Neither of these treatment options target the foundational issue of ECM cartilage breakdown in the intervertebral discs. By age 35, 30% of people show signs of DDD; by age 60, this increases to 90% (2). That we are aware of, this is the first perinatal tissue allograft in the medical marketplace that may be applied in a homologous fashion per FDA 361 guidelines to replace or supplement missing or damaged connective tissue. All other non-surgical paradigms focus on symptom management and do not address the disc’s collagen structural degeneration. In collaboration with medical providers across the country, we are actively investigating additional homologous use applications for this technology in tissue defects associated with the load-bearing joints of the knee, hip, shoulder, spine, ankle, and foot.
Billions of dollars are spent annually on the surgical care and treatment of patients suffering from degeneration of load-bearing joints and intravertebral discs. We are honored to offer patients evidence-based and structural tissue defect-specific non-surgical applications on a global scale.
Additional Sources:
About Regenative Labs: Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with daily challenges of innovations in healthcare, the company provides non-addictive, non-invasive options for patients. Regenative Labs’s expert product research and development team complies FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines. Learn more at Regenative’s website: www.regenativelabs.com
SOURCE Regenative Labs
Aram Avila-Herrera, James B. Thissen, Nisha Mulakken, Seth A. Schobel, Michael D. Morrison, Xiner Zhou, Scott F. Grey, Felipe A. Lisboa, Desiree Unselt, Shalini Mabery, Meenu M. Upadhyay, Crystal J. Jaing, Eric A. Elster & Nicholas A. Be

Ana Paula C. Jalkh, Aziza K. Eastmond, Chaitra Shetty, Syed Muhammad Hannan Ali Rizvi, Joudi Sharaf, Kerry-Ann D. Williams, Maha Tariq, Maitri V. Acharekar, Sara Elena Guerrero Saldivia, Sumedha N. Unnikrishnan, Yeny Y. Chavarria, Adebisi O. Akindele, Pousette Hamid



If liquids make their way into the bandage, the gauze along its perimeter will turn a dark blue color to indicate a new bandage is needed.

A comprehensive atlas of microRNA and messenger RNA expression in healing and non-healing human wounds highlights possible new approaches for treating venous ulcers – a common type of chronic, non-healing wound.


Simona Serini and Gabriella Calviello
New Financing to Meet Significant Growth in Global Demand for MolecuLight’s i:X® and DX™ Point-of-Care Imaging Devices for the Wound Care Industry
TORONTO, Aug. 11, 2022 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging for real-time detection of wounds containing elevated bacterial loads, announced that it has completed a financing with BDC Capital and iGan Ventures. The funds are to support MolecuLight’s continued global expansion to meet growing customer demand for its MolecuLight i:X® and DX™ devices. Leonard Kofman and Jody Staggs, Managing Director of SWK Holdings will join MolecuLight’s Board of Directors as observers.

MolecuLight receives financing from iGan Partners and BDC Ventures (CNW Group/MolecuLight)
“With the continued growth in global demand for our i:X and DX platforms, we are happy to announce this financing from BDC Capital and iGan Ventures, who has been an early investor in MolecuLight,” says Anil Amlani, CEO of MolecuLight Inc. “The proceeds will support the continued growth of our commercial operations and infrastructure to meet market demand”.
“We have invested in MolecuLight since inception and are thrilled to see the company achieve global commercial success,” says Sam Ifergan, Founder and President of iGan Partners. “Their customers continue to generate a wealth of published data showing the improved outcomes and cost savings, which is supporting MolecuLight becoming the standard-of-care in wound care globally”.
“BDC is proud to participate in the financing of MolecuLight, an impressive Canadian company that is making a global impact in terms of improving healthcare outcomes,” says Leonard Kofman, Partner with BDC Capital’s Intellectual Property-Backed Financing practice. “MolecuLight solved an unmet clinical need – the need to detect bacterial burden in wounds, and has commercialized a suite of products that is positively impacting wound care globally. Demand for the technology is strong and growing and we believe the company is well positioned for continued growth and success”.
The MolecuLight devices are sold in North America through its direct sales and clinical applications team and internationally through MolecuLight’s 15 specialized distributors in 18 countries.
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which include two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
Tungsten Advisors served as the exclusive financial advisor to MolecuLight Inc.
About BDC Capital
BDC Capital is the investment arm of BDC, Canada’s bank for entrepreneurs. With over $3 billion under management, BDC Capital serves as a strategic partner to the country’s most innovative firms. It offers a full spectrum of capital, from seed investments to transition capital, supporting Canadian entrepreneurs who wish to scale their businesses into global champions. Visit bdc.ca/capital.
About iGan Partners:
Based in Toronto, iGan Partners is Canada’s leading health technology investors focused on disrupting the sector through breakthrough innovation that dramatically improve patent are while reducing costs. The firm focuses on identifying at an early-stage, then commercializing and scaling technological advancements in AI/Cloud-enhanced medical devices and digital health. iGan provides portfolio companies with smart-capital, active support, and access to a network of industry partners and sector-specific co-investors to help them grow and succeed.
About Tungsten Advisors:
Tungsten Advisors (www.tungstenadv.com) is an investment banking firm focused on strategic advisory and corporate finance for healthcare and technology companies. Tungsten provides transactional services including financings (private placements/PIPEs), corporate licensing and mergers and acquisitions (M&A). Tungsten also focuses on company incubation and makes direct investments alongside the creation of new companies in healthcare and technology.
Securities offered through Finalis Securities LLC Member FINRA/SIPC. Tungsten Partners LLC d/b/a Tungsten Advisors and Finalis Securities LLC are separate, unaffiliated entities.
SOURCE MolecuLight

A Preliminary, Retrospective Comparative Study Kaissar Yammine, Sandra Akiki, Chahine Assi, Fady Hayek

Catherine T. Milne, MSN, APRN, CWOCN-AP Amanda Estapa, ACNP-BC, CSW, FACCWS

Harry Schneider, DPM, FACFAS

Diane L. Krasner, PhD, RN, FAAN, FAAWC, MAPWCA
Future applications for the early, preventative use of amniotic membrane allografts in addition to the current standard of care for DFUs present a novel opportunity to reduce long-term morbidity and amputation risk in diabetic patients.
PENSACOLA, Fla., Aug. 8, 2022 /PRNewswire/ — A new MDPI study, co-authored by Regenative Labs signifies a huge win for patients suffering from diabetic foot ulcers (DFUs). DFUs are debilitating for an individual. They are painful, long lasting, and, even with proper care, can lead to amputation. The current standard of care for DFUs is debridement, the medical removal of dead, damaged, or infected tissue to improve the healing potential of the remaining healthy tissue. The increased healing time and augmented risk of amputation associated with the current standard of care only solidifies the need for new DFU treatment alternatives. Regenerative medicine is such an alternative.
With approximately 37.3 million diabetic adults in the United States, physicians are faced with an epidemic. Given the projected increase of 1.4 million new diagnoses of diabetes mellitus each year, advancing knowledge and care for the disease and its related conditions is especially relevant.
Regenative Labs’ AmnioText™, a dehydrated amniotic membrane allograft, was used to close a grade 5 wound according to the Curative Health Services (CHS wound grade scale), described as full thickness and subcutaneous tissues, exposed tendons, ligaments, and/or joints, plus necrotic tissue in the wound, in 7 weeks. Grade 5 wounds have a 91.5% rate of not healing at all. This unsettling outcome emphasizes the efficacy and importance of amniotic membrane allografts in revolutionizing the standard of care for DFUs.
The inability to heal DFUs presents a severe danger to patients as anywhere from 5 to 24% of untreated DFUs can lead to limb amputation within 6–18 months. These infections can lead to long-term impairment and possible lower-limb amputation without timely and correct management.
“Regenative Labs and our products are focused on ameliorating this problem with our outcomes-based approach. We provide the highest quality amniotic membrane allograft to allow doctors to provide predictable outcomes for their patients,” shared Regenative Labs CEO, Tyler Barrett.
Amniotic membrane allografts, such as those provided by Regenative Labs, have proven to augment the body’s ability to regenerate the structural tissue defects associated with DFUs; they are also comparable in cost to the standard of care, which averages about USD 17,245. Not only is the current standard of care for DFUs less effective, but it is also high in cost and typically relies on inpatient expenditures.
Medicare now recognizes the medical necessity of amniotic membrane allografts in the treatment of both DFUs and venous stasis ulcers. Consequently, many patients rely on Medicare to assist with the costs associated with DFU treatment. This presents the opportunity for human amniotic membrane allografts to be utilized in rural and underserved communities where DFU treatment is typically delayed due to high costs and a lack of supplies associated with traditional treatment. This could exponentially decrease the risk of amputations in diabetic patients in these rural and underserved communities.
About Regenative Labs: Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with daily challenges of innovations in healthcare, the company provides effective, non-addictive, non-invasive options for patients. Regenative Labs has a laser-focused, expert product research and development team which follows FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines.
Learn more at Regenative’s website: www.regenativelabs.com
SOURCE Regenative Labs


A Retrospective Study from Kexin Li, MD


Pressure Ulcers Educational sessions specifically for people working in industry who want to refresh/upgrade their knowledge of skin health and wound management


Rachel Torkington-Stokes, MSc, BSc(Hons)


Garber, Susan L. MA, OTR, FAOTA, FACRM; O’Sullivan-Drombolis, Deirdre BScPT, MClSc (Wound Healing)

Our educational sessions promote the best practices in skin health and wound healing



Following the Science and Consensus Guidelines

David D Zabel, MD, FACS


Experience at a Rural Hospital

Wound Professionals for Use From Day 1

A Retrospective Observational Study


September 7 – September 10

Xianjie Yang, Huan Wang, Zhiqiang Song, Qiquan Chen Department of Dermatology, Southwest Hospital, Army Medical University, Chongqing, People’s Republic of China

Zahraa Mansoor, Ali Modaweb


Anna Brunauer, René D. Verboket, Daniel M. Kainz, Felix von Stetten, and Susanna M. Früh
PENSACOLA, Fla., Aug. 1, 2022 /PRNewswire/ — Regenative Labs, a leading HCT/P manufacturer, and AAPC, the nation’s largest medical coding, training, and certification association, are pleased to announce a strategic healthcare compliance collaboration. By combining AAPC’s medical training and credentialing expertise with Regenative Labs’ human tissue allografts, together both organizations will support the pioneering of the first Wharton’s jelly allografts to be assigned a Q code and be approved for application directly to a defect using a syringe.
“This is an important step in furthering the mission of educating providers and elevating healthcare outcomes and we’re proud to be a part of it,” shared AAPC CEO, Bevan Erickson.
Regenative Labs received approval from the Centers for Medicare & Medicaid Services (CMS) to cover CoreText™ and ProText™, the first Wharton’s jelly allograft products recognized as a 361 HCT/P by CMS regulated under 21CFR 1271.10, establishing a new Level II HCPCS code Q4246 “CoreText or ProText, per cc.” According to CMS, both solutions provide the extracellular matrix needed for the infiltration, attachment, and proliferation of cells required to repair damaged tissue. They are typically used for muscle and cartilage tears and help repair damaged tissue due to wounds and tissue defects and are applied directly to the defect using a syringe.
Regenative Labs, now seeking to collaborate to further HCT/P compliance, recognizes the value AAPC brings to the healthcare industry as the world’s leading healthcare association, with more than 200,00 members and 30+ years of supporting healthcare professionals, providers, payers, and health systems
AAPC will deliver healthcare providers important coding resources, including full skeletal illustrations and applicable DX codes for Regenative Labs Wharton’s jelly allografts products for specialties, including Orthopedics, Pain Management, Podiatry, and Rheumatology. In addition, AAPC will create a customized web-based training module to ensure providers understand how to utilize more specific homologous applications with current DX codes to support accurate procedural reimbursement for these products.
“We look forward to the clarity this collaboration will bring to the market allowing proper documentation of homologous use applications,” said Regenative Labs CEO Tyler Barrett.
Regenative Labs firmly believes that no other organization understands coding regulations and documentation requirements better than AAPC, leading AAPC to be the right partner for this important project.
About AAPC: AAPC’s mission is to advance the business of healthcare by providing professional training, industry-standard certifications, and comprehensive solutions to individuals and organizations across medical coding, billing, auditing, compliance, and practice management. As the most trusted source for driving accuracy, profitability, and peace of mind, AAPC helps healthcare organizations reach the full potential of their revenue cycle. Learn more at AAPC’s website: www.aapc.com
About Regenative Labs: Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with daily challenges of innovations in healthcare, the company provides effective, non-addictive, non-invasive options for patients. Regenative Labs has a laser-focused, expert product research and development team which follows FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines. Learn more at Regenative’s website: www.regenativelabs.com
SOURCE Regenative Labs
This article was originally published here
Heba Tallah Mohammed, Robert L. Bartlett, Deborah Babb, Robert D. J. Fraser, David Mannion


by Elizabeth Hogan Hamacher, MHL, BSN, RN, VP of Clinical Services and Support,


A Systematic Review of Clinical Evidence and Future Directions
Learn about wound care and healing wounds with hyperbaric oxygen therapy.
Hosted by Brian Finestein, Chief Executive Officer of Saint Clare’s Health, ‘Let’s Talk Health’ is a FREE ONLINE community series which provides accurate, trusted, and current information on health issues.





More than one-third of U.S. adults gained 10 percent body weight or more over the last 10 years, according to a study recently published in the Journal of Obesity

A Swedish study has found two sodas a day could double the risk of diabetes – even if they are diet versions.

The Tumor suppressing protein P53, already known as the guardian of the genome for its cancer-fighting action by which it blocks cell division in case of malignancy, is now also getting attention for its role in tissue repair in a study by molecular biologists from the universities of Bristol and Cambridge

Wound healing is highly regulated, but oxidative stress (OS) can disturb this healing process in chronic wounds.

into wound healing products

topical gene editing to close complex cutaneous wounds

among Nurses Working in South Wollo Zone Government Hospitals, Ethiopia

for its Synthetic Resorbable Hybrid-Scale Fiber Matrix, Restrata®
Joins Cerner Code Program
WoundZoom Digital Wound Management solution now available in Cerner’s App Gallery
STEVENS POINT, WI – July 27, 2022 – Perceptive Solutions, Inc., developer of WoundZoom Digital Wound Management, today announced its integration partnership with Oracle Cerner and the availability of WoundZoom in the Cerner Code Program App Gallery. This partnership provides a secure and reliable exchange of wound care data between WoundZoom and a facility’s Cerner EHR system.
Perceptive Solutions joins the Cerner Code Program as a trusted integration partner so our customers can leverage the benefits of our validated application, WoundZoom, while eliminating additional steps in their workflow. Data captured using WoundZoom at the bedside, such as precise wound measurements, images, and clinical assessments automatically sync to patients’ charts, creating a more efficient workflow and a complete patient record in the EHR.
“Our innovative digital wound management solution enables clinicians to spend more time with patients through automated charting, wound imaging, and elimination of the manual measurement process. Cerner is a leading EHR provider for inpatient facilities and we are excited to provide accessibility of WoundZoom to Cerner customers,” said Mark Lacerte, President of Perceptive Solutions. “The technology integration through Cerner’s validation process enables a secure and reliable data flow from our solution into Cerner’s clinical charts. This enables healthcare facilities to more efficiently share valuable wound care data between both clinical and administrative team members within their EHR.”
About Perceptive Solutions
Perceptive Solutions modernizes the practice of wound care with technology-enabled systems designed to increase clinical efficiency, improve care quality, and mitigate risk. Integrating smoothly with your EHR, WoundZoom utilizes the latest AI and imaging technology to capture accurate wound images and measurements from your smart device, automatically prompt and document appropriate actions, and create a continuous, standardized clinical record across shifts, floors, and facilities. For more information, visit https://perceptivesol.com/.
Media Contact
Karen Guzdzial
Director of Marketing
(727) 225 7944
karen.guzdzial@woundzoom.com

A Comprehensive Review of Literature


Matching Advanced Wound Care Therapy to Wound Conditions







That day I had operated on a patient who was suffering with a non-healing ulcer on his leg for a long time, then followed up on my patient in ward suffering with bed sores and another patient with diabetic foot wound

It is intended to pave the way for improving precision-based medicine when treating diabetic foot ulcers.




Study Confirms the Utility of MolecuLight to Inform Clinicians to the Presence and Location of Clinically Significant Bacteria and Improves Treatment Plans & Outcomes over Conventional Diagnostic Methods
LEEDS, UK and TORONTO, July 13, 2022 /PRNewswire/ – MolecuLight Inc., the leader in fluorescence imaging for detection and localization of elevated bacterial load in wounds, announced the publication of an independent, blinded randomized controlled trial in Diabetes Care. The publication on this 56-patient trial, titled “The use of Point-of-Care Bacterial Autofluorescence Imaging in the Management of Diabetic Foot Ulcers: A Pilot Randomized Controlled Trial“1 reported that the use of a MolecuLight i:X® device to visualize the presence of elevated bacterial burden in wounds doubled 12-week wound healing rates (204%) in diabetic foot ulcer patients over standard-of-care alone.
Diabetes is a significant global health ailment: over 416 million people have diabetes worldwide2 and 25% of these patients develop a diabetic foot ulcer (DFU)3, greatly diminishing quality of life and increasing the need for costly and extended treatment. In the UK, the NHS spends £1 billion ($1.25 billion US) annually on DFU care and management24.
“As a clinician in wound care, especially when managing patients with chronic wounds, the holy grail is improvement in wound healing rates”, says David Russell, Associate Professor in Vascular Surgery at University of Leeds and lead author in the study. “In our randomized controlled trial, the results were impressive – the use of a MolecuLight device to inform our wound care decision-making helped us double the number of wounds that were healed at 12 weeks. This has benefits for the patient and our healthcare system.”
Patients were stratified into two groups, one in which the MolecuLight device was not used, and one in which clinicians used the MolecuLight device bi-weekly to assess diabetic foot ulcers for the presence of elevated bacterial burden. For the MolecuLight group, fluorescence imaging was performed after treatment. Fluorescence indicated the presence of elevated bacterial burden in over 80% of the wounds. Additional treatment based on imaging findings was performed as the discretion of the clinician, and most often included further debridement focused on the regions with elevated bacterial loads. Importantly, there was no increase in antibiotic prescribed in the MolecuLight group.
Alongside the impressive 2-fold improvement in healing rates, this study showed an association between baseline fluorescence and wound outcomes. Of the patients with negative fluorescence images at the baseline visit, 53.9% healed at 12-weeks, versus 37.5% with positive baseline fluorescence images. In other words, patients were 36% less likely to heal at 12 weeks if their wound was positive for high bacterial loads at the beginning of their treatment, as depicted by MolecuLight. Wound area reduction was superior in the MolecuLight arm and patient quality of life diverged toward improvement in the MolecuLight arm at 4 weeks and toward deterioration in the control arm at 12 weeks.
“To improve decision-making and care with DFU patients we must be able to measure what we manage. The MolecuLight i:X, as illustrated by the results in this RCT, is a powerful tool for screening DFUs for infection as well as monitoring new or worsening bacterial burden over time”, says David G. Armstrong, Professor of Surgery, Director of the Southwestern Academic Limb Salvage Alliance (SALSA) at Keck School of Medicine of the University of Southern California as well as the US-appointed delegate to the International Working Group on the Diabetic Foot (IWGDF). “This new study provides further data for the improved healing rates and improved patient care that can be achieved in a clinic with routine use of fluorescence imaging to detect wound bacteria.”
“We congratulate Dr. Russell and the team at Leeds for their excellent study and publication that shows the utility of MolecuLight to detect elevated bacterial burden and to inform clinical decision-making at the point-of-care”, says Anil Amlani, MolecuLight’s CEO. “A doubling of 12-week wound healing is a significant outcome and is consistent with what thousands of wound care clinicians are experiencing worldwide, that MolecuLight enables clinicians to deliver superior, proactive bacterial/infection management that improves wound outcomes”.
The Leeds Diabetes Limb Salvage service is now using the MolecuLight device to image all patients with wounds that are failing to achieve a healing trajectory within 4 weeks. To help manage patient volumes, patients who are negative with MolecuLight are triaged, and are then referred to community care as their wounds are considered manageable and able to achieve a healing trajectory.
This new RCT is part of a broad body of clinical evidence showing the many benefits of the MolecuLight i:X and DX devices across the range of wound care applications to help inform and improve clinical decision-making. This list of clinical evidence includes over 60 peer-reviewed publications and 1,500 studied wound patients.
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercially released devices, including the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, provide point-of-care handheld imaging devices for the global wound care market for the real-time detection and localization of elevated bacterial load in wounds and for digital wound measurement. MolecuLight procedures performed in the United States can benefit from an available reimbursement pathway including two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant unmet needs including food safety, consumer cosmetics and other key industrial markets.
For more information, contact:
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
T. +1.647.362.4684
rsandler@moleculight.com
www.moleculight.com
The platform serves as a comprehensive resource for podiatry news and information to aid clinical decision-making and improve patient outcomes
MALVERN, Pa., July 12, 2022 /PRNewswire-PRWeb/ — As healthcare professionals across the podiatry community manage an ever-changing and complex field, HMP Global, the leading healthcare event and education company, today announced the launch of two value-added offerings, PodiatrySource and the Podiatry Learning Network.
The Podiatry Learning Network serves as the premier digital hub for podiatrists and offers direct access to clinically relevant news and information, expert perspectives, and continuing education. The network joins HMP Global’s portfolio of well-respected learning platforms dedicated to serving as online information hubs, and is a singular destination offering practical, relevant content and education guided by podiatry professionals.
Visitors to the new network can create a customized experience, selecting topic preferences most pertinent to them; receive regular e-mail updates with breaking news and carefully created content; grow professionally with the network’s repository of education; and connect with other podiatry colleagues around the world through the platform’s networking options.
“With the creation of the Podiatry Learning Network, HMP Global is delivering a single, definitive resource that podiatry professionals can rely upon to access important clinical knowledge on new therapies and treatments on a broad spectrum of topics, and enjoy a highly specialized content experience,” said David DePinho, President, HMP Global. “Healthcare professionals in the podiatry field will benefit from this comprehensive resource to receive ongoing professional development and provide the highest quality of patient care.”
PodiatrySource
HMP Global is also launching PodiatrySource, a one-stop resource with independent, clinically reviewed, and unbiased information to help clinicians make informed decisions about podiatry care products.
Powered by Podiatry Today, the award-winning, premier publication and online platform, PodiatrySource will reach buyers with the trusted information they need to act and make decisions about product selection and purchasing. It will provide 24/7 access to information on an interactive, user-friendly digital platform.
“The Podiatry Today audience is invested in providing patients optimal care and staying abreast of the latest product offerings across the field is part of how they achieve this goal,” said Jennifer Spector, DPM, FACFAS, Managing Editor of Podiatry Today, a trusted educational resource in podiatric medicine and surgery that provides engaging content to help clinicians improve patient care. “Creating PodiatrySource is a natural synergy of the education provided by Podiatry Today, with robust information about innovations provided by companies in the podiatric space.”
PodiatrySource currently highlights products focused on the office-based practitioner, and future updates will include more products and companies to best serve the full foot and ankle medical and surgical community, Spector said. PodiatrySource is open access with no subscription required for use. Industry contacts may submit product information through the website, and enhanced opportunities are available.
“PodiatrySource is designed to quickly become podiatry’s most relied upon tool to navigate the many options for products and services available to healthcare professionals in the podiatry community,” DePinho said. “It offers credible, trusted, non-biased clinically reviewed content that clinicians can use during the decision-making and purchasing processes.”
The new PodiatrySource resource guide is modeled after the highly successful WoundSource product reference guide. First published in 1998, WoundSource now includes more than 1,700 products manufactured by more than 200 wound care companies. Its digital companion, woundsource.com, includes product lists and reviews as well as articles, blogs by industry thought leaders, white papers, and other educational resources for wound care professionals.
For more information about PodiatrySource and the Podiatry Learning Network, visit hmpgloballearningnetwork.com/site/podiatrynet.com.
ABOUT HMP GLOBAL
HMP Global is the force behind Healthcare Made Practical — and is an omnichannel leader in healthcare content, events, and education, with a mission to improve patient care. The company produces accredited medical education events — in person and online via its proprietary VRTX virtual platform — and clinically relevant, evidence-based content for the global healthcare community across a range of therapeutic areas. Its brands include the HMP Global Learning Network, healthcare’s most comprehensive source for news and information; Psych Congress, the largest independent mental health meeting in the U.S.; the Evolution of Psychotherapy, the world’s largest independent educational event for mental health professionals; the Leipzig Interventional Course (LINC), the leading, global gathering for interdisciplinary cardiovascular specialists; EMS World Expo, North America’s largest EMT and paramedic event; and the Symposium on Advanced Wound Care (SAWC), the largest wound care meeting in the world. For more information, visit hmpglobal.com.
Media Contact
Sandi Beason, APR, HMP Global, (601) 573-1737, pr@hmpglobal.com
SOURCE HMP Global




and Increase Communication Across Inpatient Complex Wound Care Teams








Wounds UK are pleased to announce the call for abstracts for the 2022 Wounds UK Annual Conference, held at the Harrogate Convention Centre, which will be held on 7-9 November 2022 at the Harrogate International Centre.
To submit your abstract use the following link: www.surveymonkey.co.uk/r/WUKH22Abstract
Poster presentations will be presented on electronic poster displays only, no hard copy posters will be on display.
Entries for the e-poster exhibition require you to submit an abstract. Every entry received will automatically be considered for the Wounds UK Award for Excellence 2022.
All abstracts will be reviewed by our judging panel, who will be looking to accept submissions that display high levels of innovation, relevance to current and/or best practice and provide high-quality research/evidence.
Award
The winner of the Wounds UK Award for Excellence will receive a free 3-day delegate pass with entrance to the gala dinner.
NANCY MORGAN
Every wound-care clinician treats diabetic patients regardless of your care point. In this course, Nancy will help you evaluate the wound, identify the best plan of treatment and steer you away from the potential setbacks for better healing rates and overall patient outcomes.
Objectives:
A National Population-Based Retrospective Cohort Study
Rosemary C Chamberlain, Kelly Fleetwood, Sarah H Wild, Helen M Colhoun, Robert S Lindsay, John R Petrie, Rory J McCrimmon, Fraser Gibb, Sam Philip, Naveed Sattar, Brian Kennon, Graham P Leese
Objective: To describe incidence of foot ulceration and amputation-free survival associated with foot ulceration status in a national population-based cohort study of people with diabetes … Research design and methods: The study population included 233,459 people with diabetes who were alive in Scotland on 1 January 2012 identified from the national population-based register (national prevalence 4.9%). Characteristics of patients identified from linked hospital and mortality records during follow-up to the end of November 2017 were compared by outcome. Cox regression was used to assess the association between history of foot ulcer and amputation-free survival … Results: The population included 23,395 people with type 1 diabetes and 210,064 people with type 2 diabetes. In total there were 13,093 (5.6%) people who had a previous foot ulceration, 9,023 people who developed a first ulcer, 48,995 who died, and 2,866 … read more
Matthew F. Kaleta, Olga E. Petrova, Claudia Zampaloni, Fernando Garcia-Alcalde, Matthew Parker & Karin Sauer
Bacteria preferentially grow as biofilm communities in diverse settings including the natural environment, industrial systems, and the medical sphere1,2,3. Growth within biofilms offers protection from adverse conditions, such as defense from protozoan grazing in the marine environments, resistance to antimicrobial agents during decontamination of industrial and medical equipment, and evasion of host immune responses during infections. Evidence of this protected mode of growth appears early in the fossil record (~3.25 billion years ago) and is common throughout a diverse range of organisms in both the Archaea and Bacteria lineages, suggesting biofilm growth to be an integral component of the prokaryotic life cycle8. Indeed, studies of biofilms formed by diverse prokaryotes have revealed common trends and phenotypic characteristics of biofilms, as addressed by several reviews. These common trends include cell-to-cell communication or quorum sensing (QS), the production of extracellular polymeric substances to form a protective matrix, the presence of eDNA … read more
Clinical and operational innovations better predict non-healing wounds and missed appointments
Further expanding its analytics capabilities and leadership position within the wound care marketplace, Net Health, a provider of specialty electronic health systems and advanced healthcare analytics, today announced the addition of two new predictive analytics resources to improve clinical and operational outcomes for wound care providers … The new Pressure Injury Deterioration Risk Indicator is built into Tissue Analytics, Net Health’s AI-powered wound imaging and analysis solution, which was recently granted breakthrough device status by the Food and Drug Administration (FDA). The Missed Visit Prediction Indicator is available in Net Health® Wound Care, one of the nation’s most widely used electronic health record (EHR) platforms for wound care. Both features are embedded in automated workflow processes and alert providers when risk is detected, enabling providers to intervene in real-time … read more
The Off-Loading Devices Market is expected to grow manifold in the upcoming period. With technological advancements like ML and AI being incorporated in abundance, the healthcare vertical is likely to reach the top pedestal in the years to come. There are Bluetooth-operated health monitors, which let doctors receive precise information, that too, from time to time … The unloading device is used to reduce physical stress on the ulcerative callus of diabetic patients, which is caused by poor circulation, lack of sensation in the foot, foot deformity or trauma. … These devices are designed to eliminate abnormal pressure points and promote healing … read more
Harrison J. Shawa, Marat Kazak, Sara Dahle, Joshua M. Schulman
Amelanotic melanoma, accounting for less than 2% of melanomas, lacks typical clinical features of melanoma and mimics other lesions, frequently resulting in initial misdiagnosis and treatment delays and contributing to a poorer prognosis compared to conventional melanoma.
Amelanotic melanoma affects both men and women and, on average, affects older individuals than conventional melanoma, with an average age at diagnosis of 62 years. Although some risk factors overlap with melanoma, individuals with amelanotic melanoma are more likely to have red hair, freckles, or sunburn easily than patients with pigmented melanoma. Other risk factors include more than 10 large nevi, plantar nevi, and a history of a penetrating foot injury or a previous amelanotic melanoma.
When occurring on acral sites, amelanotic melanoma may mimic a variety of benign entities, including verrucae, calluses, poromas, hematomas, foreign bodies, fungal infections, blisters, ulcers, and pyogenic granulomas. We herein report a case of an acral amelanotic melanoma … read more
New data has been published today (July 7) evidencing findings that could lead to better treatment for people with lethal lung infections and infected diabetic foot ulcers caused by antimicrobial resistant (AMR) bacteria … Clinical stage biotechnology company, Destiny Pharma plc., focused on the development of novel products to prevent life-threatening infections, revealed the publication of the new data on XF-73 with Cardiff University in Frontiers in Cellular and Infection Microbiology a peer-reviewed publication … read more
Zahra Rajabloo, Mohammad Reza Farahpour, Parvaneh Saffarian & Saeed Jafarirad
Skin wounds cause damage to healthcare systems and loss economic. Wounds are classified as acute and chronic based on the pathogenesis and consequences. Acute wounds induce molecular processes to obtain structural integrity. Immune cells and factors play pivotal roles in acute wound healing3. The faulted regulation of the immune response results in the formation of chronic wounds. Infectious wounds are a form of acute wounds characterized by the presence of bacteria in viable tissue and damage to tissues. The infections start with bacteria colonization and can cause systemic infection. Staphylococcus aureus and Pseudomonas aeruginosa are the most common bacteria in infected wounds. In infected wounds, the wound healing process is delayed. Infected wounds also cause overproduction of reactive oxygen species and induce faults in antioxidant systems … read more
Regenative Labs CEO predicts this will have significant implications to advance multiple fields.
PENSACOLA, Fla., July 8, 2022 /PRNewswire/ — Regenative Labs, a leading FDA-registered and HTC/P manufacturer, confirms that the Institutional Review Board (IRB) of the Institute of Regenerative and Cellular Medicine (IRCM), an independent body which facilitates and validates regenerative and cellular medicine through collaborative and translational research, has approved its protocol for observational data of homologous use applications for Regenative’s products.
In noting receipt of the IRCM IRB letter, CEO Tyler Barrett observed that this is a key step in facilitating other studies for use of Regenative’s products. “We believe this will allow clinicians to explore new homologous use applications and make it easy to follow their patient outcomes,” he said, adding, “We are committed to advancing this field of research.”
The IRB letter confirms a key classification, known as 361, allowing Regenative to further studies for the use of its products.
“We are excited that this will allow us and our partner physicians to look for new uses,” said Barrett, pointing out that connective tissue is found throughout the body and that while the current use is heavily concentrated in addressing defects in the knee and specialties like orthopedics, Regenative has received queries and reports from specialties like podiatry and urology for cases of wound care and urinary incontinence.
In confirming receipt of the IRB’s letter, Regenative Labs confirmed their continued adherence to applicable state, local and federal regulations and requirements. They also look forward to a strong relationship with regulators.
The specific observational data collection approved is through MedngineTM of homologous use applications using ProTextTM, CryoTextTM,SecreTextTM, CoreTextTM and AmnioText.
Protocol number: RL-ME-002
IRB approval number: IRCM-2022-311
About Regenative Labs : Regenative Labs produces regenerative medicine products to address the root cause of a patient’s conditions using Wharton’s Jelly innovations rather than masking the pain with other treatments. Regenative Labs works closely with scientists, physicians, hospitals, and surgery centers to constantly monitor and improve patient progress and outcomes for new product development. Formed by veteran industry professionals familiar with the daily challenges of innovations in healthcare, the company provides effective, non-addictive, non-invasive options for patients. Regenative Labs has a laser-focused, expert product research and development team which follows FDA guidelines of minimal manipulation for homologous use. The company adheres to AATB and FDA guidelines. Learn more at: https://regenativelabs.com.
SOURCE Regenative Labs
This article was originally published here
ActiGraft is an FDA-cleared wound care solution that enables health care providers to produce—in real time—in vitro blood clots from a patient’s whole blood. Once applied, the blood clot tissue serves as a protective covering and supports wound healing processes that naturally occur in the body. Testimonial: “I endorse ActiGraft as it uses the body’s own healing cascade to help initiate the wound healing process and has a unique role as a topical dressing in the wound care space.” —Dr. Bryan Doner, DO, D&P Medical Group … read more
Sarah Gardner and Sweta Rai
This session will help you understand the diversity and complexity of leg ulcers and also the management of unusual causes of leg ulcers. She aims to expand your knowledge base on different presentations of leg ulcers and will talk about evidence based management and newer techniques of leg ulcer management.
Dr Rai has an interactive and inclusive style of speaking and therefore questions and discussion will be very welcome during and after her talk … read more
Vegan diets have gained a lot of popularity around the world, mostly in America. About 6% of Americans claim that they are vegans. That is a 600% increase in veganism from 2017 to 2021. There are many reasons that people nowadays are adopting a vegan diet and it includes health and ethical reasons. One of the health reasons that this review refers to is type 2 diabetes. There have been previous studies of what a vegan diet has on the health and the prevention of metabolic syndrome. There have been studies that showed that the risk of cancer can be reduced on a vegan diet, but is still under debate that this is the main reason for it. The review aimed to show studies between vegan diets and see if there is a reduction in risk for type 2 diabetes … read more
Biofilm: A complex microbial community containing bacteria and fungi. The microorganisms synthesize and secrete a protective matrix that attaches the biofilm firmly to a living or non-living surface. The biofilm contributes to underlying wound infection, chronic inflammation, and delay in healing, and it is present in 80% to 90% of chronic wounds and 6% of acute wounds.
Epibole: Rolled or curled-under closed wound edges. These rolled edges are thickened epidermis that may be callused, dry, scaly, and/or hyperkeratotic. When epibole is present in a wound, it signals to the body that the wound has healed, even though the wound remains open. Epibole must be resolved to allow the wound to close … read more
Aim This study was conducted as a randomised controlled study to determine the effect of tea tree oil on acute wound healing.
Yeliz Sürme, Gülsüm Nihal Çürük, Ayça Lekesizcan and Saim Özdamar
Methods Rats were divided randomly into two groups, non‑diabetic and ‘diabetic’; rats in the diabetic group were made diabetic by intraperitoneal streptozotocin induction at 50 mg/kg. Each group was then subdivided into sunflower oil, tea tree oil and saline (0.9% NaCl) groups. After incisional wound formation, rats were wound-dressed according to their treatment group every day for 15 days. On day 3, 7 and 15 following the wound formation, 0.5cmx0.5cm full thickness tissue samples were taken and examined histopathologically.
Results On day 3, the epithelisation and inflammatory cell density of the non‑diabetic tea tree oil group was found to be statistically significantly higher than the diabetic saline group. There was a statistical difference in favour of the non‑diabetic tea tree oil group in terms of procollagen and mature collagen density. In addition, the non‑diabetic tea tree oil group had a statistically higher angiogenesis amount than the diabetic and non‑diabetic saline and the diabetic sunflower oil groups on day 15 (p<0.05).
Conclusions It has been determined that tea tree oil has an accelerating effect on wound healing and is an alternative method that can be used in wound dressing … read more
admitted to two public tertiary referral hospitals in Australia
Sarah M Manewell, Sarah J Aitken, Vanessa L Nube, Anna M Crawford, Maria I Constantino, Stephen M Twigg, Hylton B Menz, Cathie Sherrington and Serene S Paul
Aims/hypothesis To identify hospital admissions and length of stay (LOS) and to investigate readmissions, cumulative LOS and associated factors for diabetes-related foot ulceration (DFU).
Methods Routinely-collected hospital admission data were used to identify DFU-related hospital admissions in two public hospitals between 2012–17. Readmission and cumulative LOS were investigated using negative binomial regression.
Results DFU-related admission was required by 749 patients. Median LOS was 8–10 days (stable across 2012–17). Readmission within 28 days was required by 62 patients (8%) and was significantly more likely with increasing comorbidities (incidence rate ratio [IRR] 1.38, 95% confidence intervals [95% CI] 1.02–1.88). Readmission within 1 year was required by 206 patients (28%), and was significantly more likely for males, unplanned admissions and increasing revascularisation requirements (IRR 1.34–1.70), and significantly less likely for those requiring minor and major amputation (IRR 0.33–0.64). The median cumulative LOS was 13 days (IQR 7–29), and was significantly longer for males, older age, unplanned admissions, those requiring dialysis, and those with increasing revascularisation requirements, comorbidities and mental health or behavioural disorders (IRR 1.02–2.30), and significantly shorter for those with more podiatry attendance (IRR 0.96, 95% CI 0.95–0.97) … read more
Jennifer Spector, DPM, FACFAS
Each year, Podiatry Today hears from leaders in the world of foot and ankle medicine and surgery about important developments and significant strides being made in the field. Here is this year’s slate of the Top 10 Innovations in Podiatry (in no particular order) that could have an impact on your patients and practice … Pedilay med (Pedilay Care) is a ready-to-use secondary foot bandage that supports foot and ankle dressings associated with multiple conditions. The company explains that the bandage could save precious care time, as one can take it on and off in a swift and gentle fashion. The bandage, made up of a biocompatible, low-friction fleece material, has thin seams and can help protect the skin … read more
Kyong Kim, DPM
By his own admission, Norbert Perez is a stubborn man. He initially rebelled against the counseling he received after a diagnosis of Type 2 diabetes four years ago.
“They started giving me pills, but they weren’t working,” says Perez, 39, a forklift operator and Rahway resident. “They told me to change my eating habits, my lifestyle.
At first, I didn’t pay any attention to it. That typical man thing we do. We just go until we drop dead.”
But when a callus blistered and his foot swelled “beyond belief,” he ended up at the Center for Wound … read more
In a recent article published by GHP (Global Health & Pharma), Kent Imaging presents SnapshotNIR, their portable and lightweight near-infrared imaging device that is replacing some outdated and cumbersome technologies of the past, pioneering the future of effective wound care solutions. Through case studies and customer stories, clinicians share their hands-on experience with SnapshotNIR and how they are providing a greater standard of care to patients with this innovative technology … read more
FEATURED PRESENTERS: Mark Portou and Amelia Swift
What makes a wound complicated? Any non-healing wound can be described as complicated, but some are more complicated than others! Complicated wounds are usually found on patients with complex medical problems. Often complicated wound aetiology is multifactorial, but outcomes are significantly worse with underlying vascular disease. Medical optimisation and risk factor modification is required to treat the rest of the individual too … read more
TORONTO, July 7, 2022 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging for real-time detection of wounds containing elevated bacterial loads, announces that it has been selected for a “Top Innovation in Wound Care 2022″ Award from Wound Management & Prevention (WMP) Journal for its MolecuLightDX™ device.

MolecuLightDX™ Wins Award as a Top Innovation in Wound Care 2022 From Wound Management & Prevention Journal (CNW Group/MolecuLight)
WMP provides news and information for professionals in wound care, ostomy care, incontinence care, and related skin and nutritional issues, and features ground-breaking research, peer-reviewed articles, and clinical discussions on topics relevant to the field. WMP and the Wound Care Learning Network are published by HMP Global, an omnichannel leader in healthcare content, events, and education.
MolecuLightDX was selected as a winner this year for its novel utility to provide a point-of-care tool to clinicians worldwide that enables the detection of elevated bacterial burden in wounds. Based on its extensive body of evidence and interviews with clinicians using it, the MolecuLight device is changing the standard of care in wound care.
“Wound Management & Prevention is dedicated to featuring the top innovations in wound care,” said Christiane Odyniec, Managing Editor. “Each July, the WMP Editorial Board nominates the newest innovations in the field of wound care, with the goal of sharing information to improve patient care. MolecuLightDX was nominated by our board for its innovation and practical applications, and we are pleased to recognize MolecuLight Inc. in this way.”
As part of WoundCon Summer 2022’s Technology Innovation Theatre, WMP will be hosting a webinar on “Wound Management & Prevention’s Top Innovations in Products & Care of 2022” on Thursday, July 14th at 1:30 PM EST. Five of the winning products will be featured, including the MolecuLightDX.
In this webinar, Dr. Charles A. Andersen, Medical Director of the Wound Care Clinic and Limb Salvage Program at Madigan Army Medical Center in Tacoma, WA will be speaking on his experience with MolecuLight and how it is changing his clinical practice. “Using MolecuLight has revolutionized our wound care practice and now allows us to provide proactive wound care,” says Dr. Andersen. “It’s a game-changer.”
Registration for the webinar is accessible here.
The MolecuLight i:X and DX devices are supported by a broad body of clinical evidence showing how they help to inform and improve clinical decision-making in wound care. This list of clinical evidence includes over 60 peer-reviewed publications and 1,500 studied wound patients.
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercially released devices, including the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, provide point-of-care handheld imaging devices for the global wound care market for the real-time detection and localization of elevated bacterial load in wounds and for digital wound measurement. MolecuLight procedures performed in the United States can benefit from an available reimbursement pathway including two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant unmet needs including food safety, consumer cosmetics and other key industrial markets.
SOURCE MolecuLight
Eric W. Temple
Subcutaneous hematomas can result from trauma. At particular risk are elderly patients with multiple co-morbidities and those who may be taking medications such as anticoagulants [1-3]. Effective treatment of subcutaneous hematomas is important in order to avoid complications including skin necrosis, infection, scarring, hyperpigmentation, tissue edema, and prolonged recovery [1-5]. Hematoma evacuation may be done using a surgical approach, in which the hematoma is evacuated under general or regional anesthesia followed by treatment with a skin graft or skin substitute [3,5]. There are several traditional dermal treatment options, including autografts, allografts, and xenografts. However, these modalities are associated with limited availability and donor site morbidity in the case of autografts, as well as potential disease transmission and immunologic rejection in the case of allografts and xenografts … read more
Dan Ziegler, Massimo Porta, Nikolaos Papanas, Maria Mota, György Jermendy, Elena Beltramo, Aurora Mazzeo, Andrea Caccioppo, Elio Striglia, Victoria Serhiyenko, Alexandr Serhiyenko, László Rosta, Ovidiu Alin Stirban, Zsuzsanna Putz, Ildikó Istenes, Viktor Horváth and Peter Kempler
Microvascular complications are responsible for a major proportion of the burden associated with diabetes contributing to substantial morbidity, mortality, and healthcare burden in people with diabetes. Retinopathy, nephropathy, and neuropathy constitute the leading causes of blindness, end-stage renal disease, and lower-extremity amputations, respectively. Since the efficacy of causal therapies of diabetic microvascular complications is limited, especially in type 2 diabetes, there is an unmet need for adjunct treatments which should be effective despite ongoing hyperglycemia. Experimental studies have indicated that diabetic microvascular complications can be prevented or ameliorated by various biofactors in animal models by interfering with the pathophysiology of the underlying condition. Some of the findings related to biofactors, like α-lipoic acid and benfotiamine, could be translated into the clinical arena and confirmed in clinical trials, especially in those focusing on diabetic polyneuropathy. Given the micronutrient nature of these compounds … read more
Patricia Inácio, PhD
Krystal Biotech is seeking U.S. Food and Drug Administration (FDA) approval of Vyjuvek (previously called B-VEC), its topical gene therapy for people with dystrophic epidermolysis bullosa (DEB). The request was made in the form of a biologics license application or BLA — a type of marketing approval — supported by data from two clinical trials that showed that Vyjuvek healed DEB wounds and kept them closed. “The unmet medical need for DEB patients remains very high and our relentless pursuit of a treatment for this disease continues with the same sense of urgency that we have always had since the founding of Krystal Biotech,” Suma Krishnan, president of research and development … read more
Aurora Piaggesi
The Living With Chronic Wounds trailer introduces a series of videos that illustrates patients’ experiences and the various challenges and approaches to wound management experienced and undertaken by wound care professionals and their organisations. The videos are produced by filmmaker Aurora Piaggesi …
Jarrod Shapiro, DPM, FACFAS, FACPM, FFPM, RCPS Glasg
Being a physician is a busy endeavor, and taking call adds both busyness and complexity to a physician’s calendar. This is doubly true when the physician is a resident, with all of the extra educational responsibilities on top of clinic, surgery, and call. As a result, being efficient is a necessity when on call. Here’s a little pearl that has helped me during call: use a call bag … read more
Marie Williams, David Davidson, Naz Wahab, Jessie Hawkins, Chinenye D. Wachuku, Robert Snyder
Diabetic foot ulcer is among the most common complications of uncontrolled diabetes and is associated with an increased risk of mortality.1 The annual incidence of DFUs worldwide ranges between 9.1 million and 26.1 million.1 It is estimated that approximately 15% to 25% of patients with diabetes will develop a DFU in their lifetime, resulting in increased incidence of hospitalizations and amputations.1,2 In the United States, the total annual medical cost for the management of DFUs ranges between $9 billion and $13 billion … read more
John C Lantis II
Hello, I’m John Lantis, vascular surgeon, and today I’d like to spend a little bit of time speaking to you about the benefits of debridement and some fundamentals of the technique. Very simple office-based debridement for the outpatient wound. The goals of debridement are to take away the skin edges that would be hyperkeratotic, which would be around the edges. Those cells are actually cells that usually don’t even know how to migrate across the wound bed and need to be removed. But one also doesn’t want to forget the base of the wound, where at the base of the wound you would have increased bacterial burden and cells, if they’re present, that are very senescent or quiescent, and that they’re not able to turn over. So there are various ways of debriding and methods, but we’re going to be talking about sharp debridement today, specifically. Sharp debridement you want to have a rounded blade; using an 11 blade … read more
Using Negative Pressure Wound Therapy With Intramedullary and Subcutaneous Antibiotic Perfusion
Keisuke Shimbo, Tatsuhiko Saiki, Haruka Kawamoto, Isao Koshima
Surgical site infection (SSI) after fracture fixation is associated with higher-grade Gustilo-Anderson open fractures (ie, type III).1,2 Patients with SSI that has progressed to deep infection or osteomyelitis must undergo multiple surgeries and may experience permanent dysfunction at the fracture site. Radical surgical debridement, orthopedic implant removal, and systemic antibiotic administration are generally performed to control SSIs. Orthopedic implant removal is considered to be an efficacious procedure. For example, 28% to 79% of orthopedic implants are removed after foot, ankle, or lower leg fracture surgery.3,4 After orthopedic implant removal, postoperative SSI rates are reportedly 0% to 20%.3-5 The standard-of-care therapeutic regimen is insufficient in the management of SSI after fracture fixation. Some studies have reported the use of negative pressure wound therapy … read more
Georgios Theocharidis, Hyunwoo Yuk, Heejung Roh, Liu Wang, Ikram Mezghani, Jingjing Wu, Antonios Kafanas, Mauricio Contreras, Brandon Sumpio, Zhuqing Li, Enya Wang, Lihong Chen, Chuan Fei Guo, Navin Jayaswal, Xanthi-Leda Katopodi, Nikolaos Kalavros, Christoph S. Nabzdyk, Ioannis S. Vlachos, Aristidis Veves & Xuanhe Zhao
Diabetic foot ulcers and other chronic wounds with impaired healing can be treated with bioengineered skin or with growth factors. However, most patients do not benefit from these treatments. Here we report the development and preclinical therapeutic performance of a strain-programmed patch that rapidly and robustly adheres to diabetic wounds, and promotes wound closure and re-epithelialization. The patch consists of a dried adhesive layer of crosslinked polymer networks bound to a pre-stretched hydrophilic elastomer backing, and implements a hydration-based shape-memory mechanism to mechanically contract diabetic wounds in a programmable manner on the basis of analytical and finite-element modelling. In mouse and human skin, and in mini-pigs and humanized mice, the patch enhanced the healing of diabetic wounds … read more
Christopher Barrett, DPM, Dot Weir, RN, CWON, CWS
Provided by HMP Education, an HMP Global Company.
For questions regarding this educational activity, please call 609-371-1137 or email accreditation@hmpglobal.com.
INTENDED LEARNERS
This activity is designed for physicians, nurses, nurse practitioners, physician assistants and podiatrists across all practice settings involved in the prevention, treatment, and management of patients with wounds.
LEARNING OBJECTIVES
After participating in this activity, learners should be better able to:
Examine the efficacy and usability of disposable mechanically powered negative pressure wound therapy
Review best practices for using dNPWT in a variety of wound types
Explore illustrative case studies using dNPWT in a variety of wound types
Robert G Smith, DPM, MSc, RPh, CPRS
This Week’s Topic: Diabetic Foot Ulcer in Complex Patient
This week’s 5MinClinChallenge contributed by Robert G. Smith DPM MSc RPh FNAP, focuses on a complex diabetic ulcer patient involving alcohol and nicotine abuse, complaining of painful neuropathy. See if you agree with Dr Smith’s approach to this difficult case … read more
Terry Treadwell
This past weekend I was invited to be part of a group of wound care practitioners from the United States, Europe, Australia, and South Africa to design a manuscript to assist health care practitioners from around the world treat hard-to-heal wounds. At the meeting, cases were presented, and treatments were discussed, and it dawned on me that everything we were discussing was information that had been discussed many times before. It seems that no matter where in the world one treats wounds, the problems are the same and solving old problems is a major issue. As one who has had the privilege to teach and write about wound treatment in Sub-Saharan Africa, for me, the old topics became new again … read more
Delthia Ricks
Scientists have engineered a synthetic biodegradable foam that can suppress inflammation, promote blood vessel growth and support the rapid healing of chronic skin wounds, an innovation that may one day improve treatment for ailing patients and possibly reduce costs of a major global health problem … The new material, tested in pigs with chronic skin wounds, matched or outperformed a clinical wound-healing product considered the “gold standard” of care. The research suggests that the investigational material may one day accelerate tissue repair among patients with skin wounds that have defied healing … read more
Intention in the Management of Pilonidal Sinus: Our Experience at a Tertiary Care Center in India
Swapnil P. Chopde, Geet R. Adhikari
A pilonidal sinus (PNS) is a small passageway in the subcutaneous tissue which develops most frequently in the sacrococcygeal area. In terms of postoperative outcomes, the decision on the best surgical treatment for PNS is still a challenge for a surgeon. Prevention of the disease recurrence and improving quality of the life can be considered primary goals of the treatment. The current study intends to compare two commonly practiced surgical treatments for PNSes-Rhomboid excision with Limberg flap repair against wide-open excision with healing by secondary intention … read more
Ayesha Marshall
Individuals with compromised skin integrity are at greater risk of skin damage or sustaining a wound, which may create a vicious circle of hard-to-heal wounds if underlying factors are present (Beeckman et al, 2020). Such hard-to-heal wounds are adding to the cumulative burden of wounds on patients, clinicians, families/carers and healthcare systems (Guest et al, 2020). Emollient therapy has been shown to reduce the risk of skin damage or sustaining a wound in individuals with fragile or at-risk skin (Wounds UK, 2015). This Made Easy aims to highlight the importance of preserving skin integrity, particularly in individuals with vulnerable skin, and how use of an emollient such as Hydromol® (Alliance) can help to reduce the risk of damage in a range of clinical scenarios … read more
NL Vasukutty, S Mordecai, A Tarik, M Subramaniam, B Srinivasan
Diabetic foot disease is associated with high morbidity and is one of the leading causes of lower limb amputation. The use of a local antibiotic carrier to augment debridement and reconstructive procedures is presented. Methods: The authors present early results of 48 feet in 47 patients from two centres in the UK. Their multidisciplinary protocol involved pre-operative assessment, debridement, culture-specific antibiotics and local antimicrobial management with an antibiotic-loaded biocomposite (CERAMENT G®, BONESUPPORT, Lund, Sweden). Of the 48 feet, 22 (46%) had various foot reconstructive procedures. Six patients had pre-operative revascularisation procedures. All patients were graded as either University of Texas 3B or 3D ulcers. Results: At a mean follow-up of 33 months (range 13–49 months), 42 feet (88%) were free of infection and 39 patients (83%) were mobilising. There were 28 wounds healed by secondary intention, 17 with primary closure and three required skin grafting. Three patients had non-healing and persisting ulcers at the most recent follow-up. Three patients had undergone below–knee amputation. The average time to wound healing was 16 weeks (range 3–24 weeks). A limb salvage rate of 94% was achieved … read more
By Joe Hannan
The mass killing at a Tulsa hospital is the most recent example of a well-documented trend of violence against US healthcare workers … Data indicate that the pandemic exacerbated the issue, and that HCPs may be underreporting violent incidents … Clinicians should report all incidents, and employers must provide adequate training and support. Pending congressional legislation could make the consequences of attacking an HCP similar to those for attacking an airline or airport worker … read more
David G. Armstrong, DPM, MD, PhD
A recent meta-analysis in the International Wound Journal took a closer look at vitamin D deficiency and patients with diabetic foot ulcers (DFUs). Lin and colleagues’ literature search initially included over 7,500 subjects with diabetes.1 They found that those subjects with diabetic foot ulcers had significantly lower levels of vitamin D, higher prevalence of vitamin D deficiency (less than 50 nmoL/L) and more severe vitamin D deficiency than those without DFU … read more
Tuba Yilmazer, PhD, RN and Hilal Tuzer, PhD, RN
The purpose of this study was to assess the effectiveness of a pressure injury prevention care bundle. Participants were 13 nurses and 104 patients cared for in the intensive care unit for at least 24 hours in a university hospital in Ankara, Turkey. The study was conducted in 2 stages: the pre-care and post-care bundle stages. In the pre-care bundle stage, the pressure injury incidence of the patients was followed by the nurses. At the end of the third month, the researcher held a 1-day training program for the nurses about the care bundle use to promote correct implementation. In the post-care bundle stage, the nurses provided care according to the bundle. Compliance with the care bundle was assessed. Pressure injury incidence rates in the pre- and post-care bundle stages were compared … read more
Curricular Integration of Take-Home Simulators in Nursing Education
Brenda Barth, Artur Arutiunian, Julia Micallef, Mithusa Sivanathan, Zhujiang Wang, Dana Chorney, Elaine Salmers, Janet McCabe, Adam Dubrowski
Simulation laboratories support the teaching and learning of required competencies and skills for professional nursing practice [1]. They provide experiential classrooms where nursing students learn and practice several skills in an environment that offers the practicality of a clinical setting without the risks to patient safety. This will be referred to as the centralized model of simulation-based education (Ce-SBE), where learners must congregate at a simulation lab to practice their skills under supervision and expert feedback using commercially available simulators … Before March of 2020, when the World Health Organization declared a coronavirus disease (COVID-19) pandemic, these specific regulated clinical skills were taught and practiced in simulation laboratories. However, during the pandemic, access to these simulation laboratories became limited due to physical distancing, and to continue skills development, other options needed to be considered [2]. This will be referred to as the decentralized model of simulation-based education (De-SBE), where learners can practice clinical, hands-on skills outside of the simulation laboratories from the comfort of their homes or other locations … read more
RESEARCH TRIANGLE PARK, N.C., June 28, 2022 (GLOBE NEWSWIRE) — About five years ago, the U.S. Food and Drug Administration (FDA) announced plans to toughen its guidelines around the development and oversight of regenerative medicine products. The goal was to make sure these therapies – which are growing rapidly in numbers – are safe and effective for consumers, while also providing a framework to encourage innovation … The changing regulatory landscape provided the impetus for Merakris Therapeutics to immediately engage with the FDA on its technology and the steps required to meet the new requirements, according to the Research-Triangle headquartered company’s CEO, Chris Broderick. Merakris was one of the first companies in its industry to do so … read more
Medtech innovation briefing [MIB296]Published: 10 May 2022
The technology described in this briefing is Granulox … Granulox is a topical sterile haemoglobin spray for managing chronic non-healing wounds … The innovative aspects are that, unlike other oxygen delivery technologies, it is designed to allow oxygen to diffuse through wound exudate … It can also be used in various settings without costly consumables, electrical power or full or partial body coverage in a chamber … The intended place in therapy would be alongside standard care for people with chronic non-healing wounds … The main points from the evidence summarised in this briefing are from 8 studies (1 meta-analysis, 2 randomised controlled trials and 5 observational studies) including a total of 530 people … Seven studies were based in the UK and are generalisable to the NHS … The evidence suggests that Granulox may improve the management of chronic non-healing wounds … Key uncertainties around the evidence are that sample sizes are small, and most studies were not randomised and had a short follow-up period … read more
By Bhavna Kaveti
Although several novel interventions were explored for wound healing, treating non-healing and chronic wounds is challenging in clinical management. In an article recently published in the journal ACS Applied Materials and Interfaces, researchers loaded RL-QN15 pro healing peptide into hollow silica nanoparticles, followed by their integration with zinc alginate (ZA) gels to form HSN@RL-QN15/ZA hydrogel … The researchers observed that the fabricated hydrogels were biocompatible, hemocompatible, porous, and exhibited antimicrobial activity against broad-spectrum microorganisms. The hydrogels showed release of bioactive RL-QN15, which was advantageous to accelerating the healing process … read more
to Identify Regions Containing Elevated Load and More Bacterial Species
New FDA Clearance Illustrates the Utility of the i:X to Reliably Detect
Clinically Significant Bacteria that Impedes Wound Healing
TORONTO, June 29, 2022 /PRNewswire/ – MolecuLight Inc., the leader in point-of-care fluorescence imaging for real-time detection of wounds containing elevated bacterial loads, announces that it has received an expansion to its FDA 510(k) clearance for the MolecuLight i:X® imaging device’s ability to detect the location of elevated bacterial loads (>104 CFU/g) in wounds. The expanded labelling also includes the device’s ability to identify areas of wounds containing more bacterial species, including key target pathogens of interest to the CDC that are major causes of antimicrobial resistance1. Detectable species include gram-negative and gram-positive species, aerobes and anaerobes. This expanded labeling is based on a detailed retrospective statistical analysis of over 350 patients.
Targeted debridement of wound using the MolecuLight point-of-care imaging device for detection of elevated bacterial burden (CNW Group/MolecuLight)
“We are thrilled with the FDA’s new clearance for MolecuLight’ ability to determine the location of elevated bacterial loads in wounds, in addition to the ability to identify regions with more bacterial species of interest”, says Anil Amlani, MolecuLight’s CEO. “Clinicians worldwide are using the MolecuLight device to visualize regions with clinically significant bacterial loads and more species of concern. With point-of-care information on bacterial load and its locations through use of a MolecuLight device, clinicians can act immediately to tailor their cleaning, debridement, antimicrobial strategies and treatments accordingly.”
This video (courtesy of Rose Raizman) illustrates the importance of visualizing the location of elevated bacterial load in a wound. In this scenario, the clinician is using MolecuLight i:X to inform their decision-making and target their wound hygiene to the areas of red fluorescence. Regions of red, indicating that the wound contains clinically significant (>104 CFU/g) levels of bacterial burden, are clearly visible on the patient’s diabetic foot ulcer (see image).
In addition, the FDA has also recognized MolecuLight’s ability to visualize regions containing troublesome bacterial species at the point-of-care. The MolecuLight device can be used to enable fluorescence-guided tissue biopsies to these regions to detect a higher number of pathogens of interest (defined by the CDC as increasing risk to develop antibiotic resistance) compared to standard-of-care-guided biopsies. The CDC has identified antibiotic resistance as “one of the greatest global public health challenges of our time”1. Strategies to combat antibiotic resistance include containing emerging threats through early detection and aggressive response and improving appropriate antibiotic use through antimicrobial stewardship programs. The expanded use of diagnostic tools, like MolecuLight, to improve accuracy and speed of pathogen detection has been called out to help improve appropriate antibiotic selection and reduce unnecessary antibiotic use1.
MolecuLight was the first to receive FDA de novo clearance for its MolecuLight i:X imaging platform and has subsequently received three additional FDA 510(k) clearances for the device.
About MolecuLight Inc.
MolecuLight Inc. is a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercially released devices, including the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, provide point-of-care handheld imaging devices for the global wound care market for the real-time detection and localization of elevated bacterial load in wounds and for digital wound measurement. MolecuLight procedures performed in the United States can benefit from an available reimbursement pathway including two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant unmet needs including food safety, consumer cosmetics and other key industrial markets.
Image: Download at: https://moleculight.box.com/s/ax8758gz0f8amouhcjjvs4xzkfa9vofo
Video: https://youtu.be/HKOCGBlIQj4
SOURCE MolecuLight
In a study of over 7,000 chronic limb-threatening ischaemia (CLTI) patients, researchers found that Black and Hispanic patients had higher three-year amputation and reintervention rates; survival, however, was higher among Black patients and similar between Hispanic and White patients. Aderike Anjorin (Duke University Medical Center, Durham, USA) delivered these findings at this year’s Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM 2022; 15–18 June, Boston, USA) … Framing the research, Anjorin stated that Black and Hispanic patients have higher rates of CLTI and suffer worse outcomes after lower extremity bypass compared with White patients. The underlying reasons for these disparities are unclear, she said, specifying that data on long-term outcomes are limited. In order to address this gap in the literature, Anjorin and colleagues examined differences in three-year outcomes after open infrainguinal … read more
MEMPHIS, Tenn.–(BUSINESS WIRE)–FedEx Corp. (NYSE: FDX) and Direct Relief continue to support those affected by the conflict in Ukraine. On Sunday, June 26, FedEx Express safely delivered 52 tons of critical medical aid to Poland from the United States via a FedEx humanitarian relief flight. This follows FedEx and Direct Relief’s first charter flight of aid for Ukrainian refugees in March and is the latest in a continuous series of shipments from Direct Relief … Aid aboard the FedEx Express Boeing 777 cargo aircraft included substantial quantities of emergency medicines and supplies, including health kits, trauma and wound care items, chronic disease and chemical exposure medications, and antibiotics. All items were provided at the request of, and approved by, Ukraine’s Ministry of Health and local Ukrainian organizations. Direct Relief team members were on site for the offload and the aid will be distributed to health facilities within Ukraine … read more
Hayley Ryan and Helen Jentz
Wounds Australia celebrated its 28th anniversary this year. Like many peak national organisations, over the course of our history, Wounds Australia has had to evolve and adapt to the ever-changing health and social landscapes in which we operate. Central to our ongoing success as a peak body is the commitment of our members, the health professionals who have chosen to dedicate themselves to the highly specialised and complex area of wound prevention, diagnosis, treatment, care, healing and research … As an organisation that is focussed on effective, real and sustainable systemic change that will result in significant improvements in the lives of Australians living with wounds, we are also dedicated to broadening the ways in which we, as the peak national body for wound prevention, treatment and healing, can support and assist those living with wounds and the families who love and care for them … read more
Allyson Y. Schwartz, Jon Bloom, M.D.
Lower limb amputations are devastating for people living with diabetes, particularly for Black Americans facing poor access to comprehensive care. A coordinated, data-driven national prevention strategy is the only way to curb this growing epidemic for all at-risk populations … When President Joe Biden rightly called out the unsustainably high price of insulin in his 2022 State of the Union Address, he wasn’t saying anything new for most industry observers. Rising insulin prices have been the subject of national conversation for years, and the push for a reasonable cap on out-of-pocket expenses has been a perennial rallying point among providers and patient advocates … Capping insulin costs is an important step, but it is just a start if we are going to stop people living with early diabetes from experiencing a worsening of their condition … The statistics and the people behind those numbers demand greater attention, smarter care, and a life free of the serious consequences of uncontrolled, complex diabetes … Consider these numbers. More than 37 million people are living with diabetes in the United States — and a further 96 million individuals have prediabetes … read more
Hemostatic Solutions for Trauma Injuries Presents Opportunities – ResearchAndMarkets.com
The “Growth Opportunities in Military Medical Device Technologies” report has been added to ResearchAndMarkets.com’s offering … Over the years, the healthcare industry has developed several unique technologies for emergency medical services and critical care. An important user of critical care healthcare products is the military or the defense forces. Advanced medical technologies have improved the care that is offered, and they also enable safer and quicker care for soldiers … Severe hemorrhages can result in hemodynamic instability, hypoxemia, multiple organ failure, or death. A key reason for patient fatality is blood loss due to trauma or injury on the battlefield. Typically, intravenous (systemic) or local (topical) hemostatic agents are used to control excessive bleeding … read more
Eli Cahan
Leafer Miller didn’t hear much after the doctor told him they had to “sacrifice the leg.” … Lying on the emergency room gurney, the self-proclaimed video game nerd and former athlete struggled to comprehend life without the leg that had propelled him on the track and to the turf for tackles on the soccer field … “It was always in the back of my mind as a worst-case scenario,” the 35-year-old Fresno, CA, native says. “But I wasn’t expecting that to be the case.”
… read more
Robin Lenz and Fahad Hussain
For the patient, the prevention of sores and injuries is better than treating them. Pressure-relieving mattresses may be essential for preventing pressure injuries (bed sores). These mattresses aid in relieving and redistributing pressure and can thereby cause a reduction of friction and shearing. Pressure-relieving mattresses provide support for the body and reduce the amount of force applied to a given area. Thus, for bedbound patients and patients who are unable to reposition themselves, these types of beds can be especially beneficial … read more
Zena Moore, Dot Weir, Shinobu Ayabe, Andrea Bellingeri, Keryln Carville, Alison Garten, Rolf Jelnes, Lee Ruotsi, Henri Post, Joanna Swan, Terry Swanson, Ewa Sturmer, Gulnaz Tariq, Kevin Woo, Michael Clark
This document seeks to help clinicians support those who do not have specialist wound training to accurately assess patients and their wounds and arrive at a broad-based, systematic rationale that will ultimately help reduce variations in clinical decision-making. The T.I.M.E. Clinical Decision Support Tool provides a structured approach to wound bed preparation … read more
Rural patients identifying as Black have more than a 10 percent absolute increased risk for major leg amputation or death compared with the overall cohort of adult Medicare patients hospitalized with a diabetic foot ulcer, according to a study published online April 21 in JAMA Network Open … Meghan B. Brennan, M.D., from the University of Wisconsin in Madison, and colleagues examined the associations of race, ethnicity, rurality, and/or neighborhood disadvantage with outcomes among U.S. patients with diabetic foot ulcers. The analysis included 124,487 patients hospitalized with diabetic foot ulcers (2013 to 2014) identified through the U.S. National Medicare Claims Data Database … read more
We (The Society of Tissue Viability) have over 100 resources, free for everyone to browse and read and share. These resources are suitable for a range of skin and wound care disciplines, roles, and educational purposes.
The university-level modules are aimed at supporting Continuing Professional Development and cover a range of topics from basic skin anatomy and physiology through to wound healing and dressing selection.
The five higher tier modules are fully endorsed by the Society of Tissue Viability.
You will need to sign up to the ActivHeal Academy to access these courses
Module 1: Anatomy & physiology of the skin
To understand how wounds heal, it is important to have a basic knowledge of the anatomy and physiology of the skin and the structures that lie within it. ESTIMATED TIME REQUIRED: 60 MINUTES
Module 2: Wound healing
After an injury to the body has occurred, healing of the wound takes place in order to restore the intact barrier provided by the skin. ESTIMATED TIME REQUIRED: 60 MINUTES
Module 3: Patient and wound assessment
This module looks at the important criteria to consider including wound assessment tools to encourage you to critically analyse established wound assessment tools used within the clinical environment. ESTIMATED TIME REQUIRED: 90 MINUTES
Module 4: Wound classification and wound management
This module will look at different wound types and the most frequently used methods by which they are staged and managed. This is meant as a guide and may compel you to look at the research behind your current practice.
Module 5: Dressing selection
As the number of wound care dressings available on the market continues to grow this course looks to ensure that the correct treatment is carried out. ESTIMATED TIME REQUIRED: 90 MINUTES
Special Report on MASD written by Elizabeth Faust, MSN, CRNP, CSWS, CWOCN-AP, MAPWCA; Siobhan McCoulough, CNS; and Patricia Idensohn, CNS
Moisture-associated Skin Damage (MASD), as the name suggests, occurs when exposure to a moisture source results in a breakdown of skin integrity. MASD is an umbrella term for a number of conditions including incontinence-associated dermatitis, peri-stomal skin damage, peri-wound skin damage and intertrigo. These conditions can impact greatly on patients so protecting the skin from damage and promoting wound healing are integral parts of skin and wound care. This area contains a variety of different resources to help aid understanding of MASD, so please sign up to our events, and explore to learn more!
… read more
Oleg Teleten, MS, RN, CWCN
Tatyana S. Polyak, MD
Jessica Espinoza, OTS
Andrew I. Li
Ariel J. Rodgers, MD
Holly Kirkland-Kyhn, PhD, FNP, GNP, CWCN, FAANP
Pressure ulcers/injuries (PU/Is) are still highly prevalent in the acute care setting. According to the Joint Commission Center for Transforming Healthcare (2021), more than 2.5 million patients in US acute care facilities are estimated to have PU/Is each year, and 60,000 die of their associated complications.1 PU/I development has many contributing factors, including prolonged pressure over bony prominence due to inactivity/immobility, shearing between skin surface and mattress/seating surface,2 poor perfusion,3 tissue ischemia, and prolonged high surface interface pressure.4-6 Incidence of PU/Is has been shown to be associated with increased disease burden, financial burden, and increased in-hospital mortality rates … read more
Biomedical engineer Amit Gefen, BSc, MSc, PhD, discusses dressings in general and gelling dressings in particular from an engineering and clinical use perspective.
Amit Gefen, BSc, MSc, PhD, is a professor of biomedical engineering at Tel Aviv University and a board member of Wound Management & Prevention. His research interests include mechanobiology, tissue engineering, cell and tissue biomechanics and applications in chronic wound research … read more
Medicare covers many products and services that are needed to manage chronic wounds. However, Medicare does not always cover the products for all indications, at the frequency that physician or the patient would like, etc. When a wound care clinic and/or practitioner believes that Medicare covers the product of service, but does not believe Medicare will cover it for a particular patient, that patient should be given an Advanced Beneficiary Notice (ABN). In addition, if a product or service, the practitioner is required to give the patient an ABN prior to treatment. If the patient is not given an ABN, and Medicare denies coverage of the product or service, the clinic and/or practitioner cannot collect payment from the patient. To help readers understand the new Medicare ABN, I interviewed Donna Cartwright, MPH, RHIA, CCS, RAC, one of new editorial board members for Today’s Wound Clinic. Donna is a professional coder who brings over 27 years … read more
In this video, Paul Kim, DPM, MS, FACFAS, discusses the clinical implications and considerations of using negative pressure wound therapy with instillation in wound care.
Paul Kim, DPM, MS, FACFAS, is a professor in the Department of Plastic Surgery and Orthopedic Surgery and is the Medical Director of the Wound Program at the University of Texas Southwestern in Dallas, Texas. For more content, visit the Negative Pressure Wound Therapy topic center … watch video
Jarrod Shapiro, DPM, FACFAS, FACPM, FFPM, RCPS Glasg
At the risk of scaring all of our readers away, I’d like to talk a bit about charting and documentation today. I know what you’re thinking: “Could there be a more boring topic?” Maybe not, but the importance of documentation is inversely proportional to how boring it is. First, remember that the chart note is your medicolegal shield. The note has to be of excellent quality, and the better the note, the better the shield. If you were a knight of the round table, would you have wanted your shield to be weak so your opponent could skewer you with their sword? Of course not … read more
Shigenori Masaki, Itaru Maeda, Takashi Kawamoto
Burn injuries are the most common type of skin injury.1 Initial evaluation of burn depth and size is essential in determining treatment strategies.1,2 Burn depth is conventionally classified into 3 main categories: superficial, partial-thickness, and full-thickness. Burn size is evaluated by estimating the patient’s total body surface area (TBSA) percentage. Minor burns, such as superficial or partial-thickness burns less than 10% of TBSA, can be managed by a primary care physician using topical agents or wound dressings in an outpatient setting.3 Full-thickness burns, however, require evaluation by a specialist in a burn center for excision and skin grafting.1-3 Clinicians occasionally encounter patients who decline operative procedures. If the full-thickness burn in such a patient is minor, conservative treatment may be acceptable.4 Few published studies have reported the use of … read more
This table illustrates common wound care services and procedures performed at hospital outpatient settings, and refers to Centers for Medicare & Medicaid Services (CMS) facility fees. For Medicare Physician Fee Schedule, see topic “HCPCS/CPT Codes Commonly Utilized in Wound Care and HBOT”.
Instructions for the interactive Tool below: 1. Select number of entries (rows) to be displayed on the table. 2. Use the “Search” field to find a specific code or description. For educational purposes only. Your hospital chargemaster, Medicare Administrative Contractor (MAC) and Coverage Determinations should serve as the definite source of billing guidelines … read more
Patients with systemic sclerosis who demonstrate digital ulcers use “significantly more” health care resources annually than patients without digital ulcers, according to data published in Arthritis Care and Research … “[Digital ulcers (DUs)] are slow to heal, specially if there is calcinosis, and can be complicated by soft tissue infections, wet and dry necrosis, eschar, underlying tissue exposure, gangrene, osteomyelitis, and amputation,” Tatiana Nevskaya, MD, PhD, of the Schulich School of Medicine and Dentistry, in Ontario, and colleagues wrote … read more
Heng Dong, Kaili Yang, Yu Zhang, Qiang Li, Weijun Xiu, Meng Ding, Jingyang Shan, Yongbin Mou
Antibiotic-resistant bacteria have become both a worldwide problem and major hidden danger that threatens global public health. Currently, the abuse of antibiotics is a particularly serious problem, often leading to the emergence of drug-resistant bacteria and even “superbacteria”, such as Staphylococcus aureus (S. aureus).1,2 Bacterial infections are very challenging to treat, as the actions and penetration of antibiotics are largely limited by the dormant lifestyle of bacteria and the extracellular polymeric substance (EPS) matrix in bacterial biofilms.3,4 Bacterial biofilms with an EPS matrix can resist host immune defenses and induce persistent inflammation, thus allowing the bacteria to become highly resistant to traditional antibiotics.5 Ineffective treatment with traditional antibiotics not only causes the rapid emergence of drug-resistant S. aureus strains but could also result in the formation of S. aureus biofilms.6 Therefore, the development of a new strategy to inhibit S. aureus biofilm formation is urgently needed … read more
By signing up to LeaRn On Demand today you will have access to:
Caitlin W Hicks, Dan Wang, Kunihiro Matsushita, B Gwen Windham, Elizabeth Selvin
Growing evidence indicates that peripheral neuropathy (PN) is common even in the absence of diabetes. However, the clinical sequelae of PN have not been quantified in the general population … To assess the associations of PN with all-cause and cardiovascular mortality in the general adult population of the United States … read more
Sibusiso Alven, Sijongesonke Peter, Zintle Mbese, Blessing A. Aderibigbe
Diabetes is a chronic condition with a high incidence of mortality and numerous complications that include diabetic foot ulcers (DFU) [1]. In 2013, it was reported that approximately 366 million individuals suffered from diabetes worldwide and in 2019, 1.5 million deaths were caused by diabetes [2]. Diabetes is a medical condition due to the inability of the pancreas to produce sufficient insulin or the inability of the body to effectively use the insulin produced [3]. Diabetic patients usually suffer from chronic injuries such as DFU and diabetic ulcers/leg ulcers. These wounds display features of a prolonged wound healing process and result in hospitalization and limb amputations [4]. About 50–70% of limb amputations are caused by diabetic injuries, and it has been reported globally that one leg is amputated every 30 s because of diabetic wounds … read more
Denise Nemeth, MPAS, CWS
Jayesh B. Shah, MD, MSc, UHM ABPM, CWSP, FAPWCA, FCCWS, FACHM FUHM, FACP
HBOT is most commonly known by health care professionals in the setting of wound healing, but did you know that the majority of the Food and Drug Administration (FDA)-approved indications for HBOT are emergency indications? As of July 2021, there are 13 approved indications for HBOT. These indications include emergencies like air emboli, crush injuries, decompression sickness, central retinal artery occlusion, idiopathic sensory neural hearing loss, iatrogenic gas embolism, gas gangrene, necrotizing fasciitis, and carbon monoxide poisoning … read more
Many people have now embraced the plant-based meat movement. Plants high in protein, such as soybeans, are common ingredients, but it’s been unclear how much of the nutrient makes it into human cells. In ACS’ Journal of Agricultural and Food Chemistry, researchers report that proteins in a model plant-based substitute were not as accessible to cells as those from meat. The team says this knowledge could eventually be used to develop more healthful products … read more
Pei Wang, Yun Wang, Yang Yi, Yan Gong, Haoran Ji, Yuci Gan, Fei Xie, Jinchen Fan & Xiansong Wang
Without an efficient and transdermal drug delivery system, patients who have skin disorders of various causes tend to experience incomplete or improper wound healing. Diabetic foot ulcer (DFU) resulting from decreased neurovascular response and multi-antibiotic-resistant bacterial infection [4] are an example of chronic wound healing. DFUs are accompanied by high morbidity and mortality and can lead to limb amputations [6] and hospitalization. Unfortunately, most treatments for diabetic skin disorders, including the application of acellular dermal matrix (ADM), electrospun nanofiber, are administered by smearing and are incapable of efficiently delivering drugs through the cuticle. Thus, to promote skin regeneration in patients with diabetes mellitus, it is necessary to develop a better drug delivery system … read more
Kelsey Millonig, DPM, MHA, AACFAS
Preventable chronic diseases currently overwhelm the US health care system. Physicians experience this firsthand daily, yet Western medicine places little priority on lifestyle modification as a treatment modality. There is not the time nor a support system in place for physicians to prioritize these concepts with patients, least of all nutritional counseling. Even if we had the time, our general medical education system does not educate physicians in-depth on nutrition.1 Instead, we must find the time to educate ourselves on these topics, and with widely available misinformation available on nutrition, we begin to question any resource … read more
During (National) Diabetes Awareness Week from 13 to 19 June 2022, Neuropad® a 10-minute pain-free screening test for the early detection of diabetic foot syndrome, a condition which can lead to serious complications such as foot ulceration, and amputation is raising awareness about this condition. Foot complications are the most feared of all the complications of diabetes, however, alarmingly, 30% of people with diabetes are unaware that foot complications are common and serious if detected late. Another sobering statistic is that the five-year mortality post amputation is worse than most common cancers and much higher than breast cancer … Nerve damage to the feet is a common complication of diabetes, but often goes unnoticed. Neuropad helps solve this problem with a simple colour change test, that provides an early warning sign … read more
Chronic wounds affect nearly 15% of Medicare patients (8.2 million people) and may cost as much as $96.8 billion per year. The most common are not venous or diabetic, even though they are the most often studied in prospective trials. The most common chronic wounds are surgical incisions that dehisce and the “wounds with no name” due to the patients’ underlying medical conditions. That is because wounds are not a disease – they are a symptom. The US Wound Registry (USWR), a 501 (c)(3) non-profit organization, has been a patient registry since 2005. Since 2014, the USWR has been recognized by the Centers for Medicare and Medicaid Services (CMS) as a Qualified Clinical Data Registry (QCDR) that collects medical and/ or clinical data for the purpose of improving the quality of patient care. While we understand that randomized, controlled trials are needed to demonstrate efficacy in a perfect world, real-world patients have an average of 6 serious co-morbid conditions and take 10 medications. These complicated patients are invariably excluded from clinical research studies, which makes it impossible to know what treatments work best … read more
In this episode, you can listen to a conversation between EWMA Podcast Host Samantha Holloway and two key figures in wound healing coming out of Scandinavia; Professor Finn Gottrup and Professor Jan Apelqvist. The discussion initiates this season’s focus on the historical development of EWMA since its inception in 1991. The major question addressed in this episode is what has changed in wound management since EWMA was established 30 years ago? The podcast provides and overview of the tremendous change the discipline of wound healing and management has went through since the foundation of EWMA … listen
Christi Druskovich, BS and Kurt A. Ashack, MHS, MD
A 73-year-old male with well-controlled rheumatoid arthritis and hyperlipidemia presented to the office for evaluation and management of multiple ulcers located on the right lower extremity. The lesions started 2 to 3 months prior as small, painful erythematous papules that began to enlarge and ulcerate. The patient denied any lower extremity swelling, related no new medications, and no changes to his current medications. He denied any trauma to the area, as well as recent illness or vaccination … read more
Author(s) : Maria Hughes, Helen Strapp, Alita Jaspar, Bram Balduyck
Venous leg ulcers (VLUs; also known as varicose or stasis ulcers) pose significant challenges to patients and healthcare systems: they are the most common type of leg ulcer, costly to manage, recurring, and may persist for months or years (Harding et al, 2015). This can significantly impact patient quality of life, leading to depression, anxiety and social isolation. Other issues associated with VLUs that can negatively impact on quality of life include the following:
The agenda has not yet been confirmed for this meeting. Please check again at a later date for more information, or call on 020 3735 8244.
… read more
We have recently been informed by our long-term funder, the NIHR, that funding for all the review groups they currently fund will terminate on 31 March 2023, and at that point Cochrane Wounds will also cease to exist. This means that we have to make difficult decisions about which reviews we will support between now and then, as our resources are limited in amount and duration.
Unfortunately, we will not be able to accept new titles and we will only proceed with high priority, high quality reviews that meet deadlines and can be produced relatively independently by author teams in an efficient way … read more
Why Do Chronic Wounds Contain Biofilm?
The process of wound healing ideally progresses from inflammation to epithelialization and, finally, remodeling. If at any point bacterial (or fungal) colonization becomes prominent, the process of wound healing is disrupted. The creation of biofilm is a microbial defense mechanism that stalls the trajectory of healthy wound healing and can contribute to the development of a chronic wound. It is estimated that 90% of chronic wounds and 6% of acute wounds contain biofilms generated by microbes.1,2 Epidemiologically, chronic wounds impact 2% of the entire US population.2 Because of this large impact, knowledge of proper wound healing and use of clinical tools to assist the wound healing process are essential … read more
Keynote Speakers: Sarah Gardner, Kristina Stiles, Paul Hardy
Free service specific/specialist days promote the best practices in skin health and wound healing … Caring for children with a chronic illness or disability can pose many challenges to healthcare professionals. Frequently this includes the risks associated with maintaining skin integrity and wound healing when faced with complex factors that can impact on patient outcomes … This advanced study day will explore some of the factors that need to be considered when assessing children with complex skin and wound care needs and will also provide insight into managing specific conditions such as pilonidal sinus, pressure ulceration and leg ulceration … read more
Kathleen Schaum, D. MS
Just when the coding for cellular and/or tissue- based products (CTPs) for skin wounds (outdated term, “skin substitutes”), had stabilized, the CMS began to inconsistently award different Healthcare Common Procedure Coding System (HCPCS) codes to new CPTs. This inconsistency has caused physicians and other qualified healthcare professionals (QHPs), hospital-wined outpatient wound/ulcer management provider-based departments (PBDs), coders, balers, and Charge Description Master directors to submit many questions to this author. Therefore, this article will answer the most frequently asked questions, which should address some of the coding confusion surrounding the application of CTPs … read more
Using Negative Pressure Wound Therapy With Intramedullary and Subcutaneous Antibiotic Perfusion
Surgical site infection (SSI) after fracture fixation is associated with higher-grade Gustilo-Anderson open fractures (ie, type III).1,2 Patients with SSI that has progressed to deep infection or osteomyelitis must undergo multiple surgeries and may experience permanent dysfunction at the fracture site. Radical surgical debridement, orthopedic implant removal, and systemic antibiotic administration are generally performed to control SSIs. Orthopedic implant removal is considered to be an efficacious procedure. For example, 28% to 79% of orthopedic implants are removed after foot, ankle, or lower leg fracture surgery.3,4 After orthopedic implant removal, postoperative SSI rates are reportedly 0% to 20%.3-5 The standard-of-care therapeutic regimen is insufficient in the management of SSI after fracture fixation. Some studies have reported the use of negative pressure wound therapy with instillation and dwell time … read more
Chronic and nonhealing wounds are a worldwide issue and are becoming more difficult to treat. In the United States alone, according to Medicare, over 8 million Americans have chronic wounds that cost the national health care system between $18.1 and $96.8 billion per year.1 If standard treatment does not adequately heal a wound, additional methods of wound care treatment may be required, and the underlying disorder must be examined to determine the need for advanced wound care modalities. Advanced wound care therapies are interventions that are used after standard wound care has failed … read more
in Rats With Experimentally Induced Diabetes
Mehmet Esat Duymus MD, Hulya Ayik Aydin MD, Abdullah Bulgurcu MD, Zeynep Bayramoglu MD, Abdullah Durhan MD, Salih Tuncal MD, Mevlut Recep Pekcici MD, Kemal Kismet MD
Red ginseng (Rg) is traditional medicine that has been used for many years in Asian and European countries, especially Korea, China, and Japan. The major components of Rg are ginsenosides, Rg1, Rb1, Rb2, and Rb3, each with its own pharmacological effect. In vivo studies have been conducted to evaluate the hepatoprotective effect of Rg in chronic liver disease and its vasoprotective effect in heart disease. In addition, an in vitro study revealed that Rg extract may stimulate wound healing by increasing growth factors on fibroblast obtained from diabetic patients. A similar effect of Rg extract has been observed on full-thickness skin wounds in rats. In another study conducted in patients with colorectal cancer, oral intake of Rg was shown to have no effect on blood cytokine levels and biochemical parameters, but oral Rg used in combination with chemotherapy regimen was found to reduce cancer-related fatigue … read more
Module 1: The Skin
This forms part of a series of modules you can access to develop your knowledge and understanding of wound care. This module is endorsed by The Society of Tissue Viability
By the end of this module, you will be able to:
This video is intended for health care professionals. It is offered for informational and educational purposes only and is not intended as medical advice. Viewer discretion is advised.
Nonabsorbable sutures or tissue adhesives used in combination with surgical strips and liquid adhesives are safe and effective means to minimizing hyperpigmentation in skin of color following Mohs micrographic surgery, according to a study … Ramone F. Williams, MD, MPhil, of the department of dermatology at Massachusetts General Hospital and Harvard Medical School, and David Ciocon, MD, of the division of dermatology at Albert Einstein College of Medicine and Montefiore Medical Center, highlighted the increasing diversity of the U.S. population … read more
Validation study to assess up to 200 adult subjects with participation from leading U.S. principal investigators.
DALLAS, June 14, 2022 (Newswire.com) – Spectral MD Holdings, Ltd. (AIM: SMD), a predictive analytics company that develops proprietary AI algorithms and optical technology for faster and more accurate treatment decisions in wound care, announces the start of its clinical validation study to support the development of its Diabetic Foot Ulcer (“DFU”) application for the DeepView® Wound Imaging System. The proprietary technology combines multi-spectral imaging and Artificial Intelligence (AI) to provide clinicians with an immediate healing assessment for DFU, which enables clinicians to provide a more accurate and timely diagnosis for therapeutic intervention.
The study will collect data from up to 200 adult subjects across seven potential clinical sites to further develop DeepView®’s AI algorithm. Patient enrollment for the validation study began this month and is expected to complete in November 2022.
Building upon promising results from the clinical training study, where the current diagnostic accuracy is 81%, the data collected from the validation study will be used to bolster the Company’s existing clinical database of DFU images and physiologic information to train and improve the DeepView® AI algorithm. Additionally, the validation study will collect data from a broader population set of up to 200 subjects to increase geographic and ethnic variability. Importantly, data collected will support the Company as it prepares FDA and CE mark submissions for DeepView®’s DFU indication, planned in 2023.
Participating investigators include: Dr. Alisha Oropallo, MD, Director of Comprehensive Wound Healing Center at Northwell Health; Dr. Brock Liden, DPM, Podiatry Specialist at Circleville Foot & Ankle Center, LLC; and Dr. Babajide Ogunllana, DPM, West Houston Foot and Ankle Center. Additional clinical sites are being evaluated and will be incorporated into the validation study in the near future.
Dr. Alisha Oropallo, M.D., FACS, FSVS, Director of Comprehensive Wound Healing Center at Northwell Health and National Principal Investigator for the study, commented: “For patients with diabetes, foot ulcers can impact their quality of life and lead to complicated infections and potential amputation. I look forward to participating in this study as DeepView® has significant potential to improve the current standard of care, resulting in faster application of advanced therapy, better wound healing and reduced overall hospital visits and utilization.”
Mary Regan, Ph.D., VP of Clinical Affairs at Spectral MD, said: “We are very pleased to have initiated the Clinical Validation Study, the next critical step towards the completion of development for the DFU application for DeepView® Wound Imaging System. To obtain robust clinical data, the Spectral MD team has selected leading wound care providers to advance and develop our DeepView® Wound Imaging technology. I look forward to working with all participating clinical sites to ensure high-quality data and clinical engagement.”
About Spectral MD:
We are a dedicated team of forward-thinkers striving to revolutionize the management of wound care by “Seeing the Unknown”® with our DeepView®Wound Imaging System.
info@spectralmd.com
This article was originally published here
The Alliance submitted detailed comments and recommendations to Novitas on its Draft LCD on Skin Substitutes for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers (DL35041) and accompanying draft Local Coverage Article (DA54117). In April oral comments submitted at Novitas’ public meeting, the Alliance had flagged – among other issues – provisions in the policy not supported by scientific evidence, as well as conficting and/or clinically incorrect policy language. In written comments, the Alliance submitted a chart (attachment A) detailing the specific provisions/policy language it flagged as problematic, the issues of concern underlying to those provisions, specific language changes to address the concern, and the clinical evidence supporting these recommendations. The chart was accompanied by additional attachments, including HCPCS and ICD-10 Codes to be added and a red-lined mark-up of suggested line edits (attachments B-E). See comments and attachments below … read more
Frank Aviles Jr., PT, CWS, FACCWS, CLT-LANA, ALM, AWCC, DAPWCA.
I’ve had the pleasure of meeting more people this year alone than at any other time in my life who’ve shared more remarkable stories about how they’ve overcome adversity … One such story comes from Captain Charles Plumb, a United States Navy jet pilot veteran who during the Vietnam War his plane was shot down over enemy territory. He ejected and was captured after parachuting to the ground. He spent six years as a prisoner of war (POW), but what really caught my attention was how thankful he is to this day that his parachute actually opened despite the nightmare that he endured. As a POW, he had to form his own method of communication although he spent most of his time alone inside an 8-by-8 cell … read more
Pherecydes Pharma (FR0011651694 – ALPHE, PEA-PME eligible), a biotech company specializing in precision phage therapy to treat resistant and/or complicated bacterial infections, today announces it will participate to the scientific symposium “Phage therapy: French experience”, which will be held on June 15, 2022 from 5:15 pm to 6:30 pm in the amphitheater A of the Palais des Congrès in Bordeaux, as part of the 23rd National Days of Infectiology (NDI).
The symposium, moderated by Dr. F.-A. Dauchy from the Bordeaux University Hospital, will host the following presentations:
Phagotherapy and IOA. Compassionate cases and clinical studies including PhagoDAIR: Pr. T. Ferry (Lyon)
Phage therapy and Infections of the Diabetic Foot Ulcer. State of knowledge. PhagoPied: Pr. A. Sotto (Nîmes)
Phage therapy and pulmonary infections. Preclinical results. Planned clinical studies: Dr. A. Bleibtreu (Paris)
Pascal Birman, Medical Director of Pherecydes Pharma, comments: “Antibiotic resistance is a major public health issue and is a central theme at this 23rd edition of the NDI. This symposium is an opportunity to highlight the interest of phage therapy through several clinical studies that will be conducted in different indications and through compassionate treatments already performed with our phages. Pherecydes Pharma and its partners are doing their utmost to ensure that these studies provide useful answers to improve the treatment of patients suffering from antibiotic resistant infections.”
About Pherecydes Pharma
Founded in 2006, Pherecydes Pharma is a biotechnology company that develops treatments against resistant bacterial infections, responsible for many serious infections. The Company has developed an innovative approach, precision phage therapy, based on the use of phages, natural bacteria-killing viruses. Pherecydes Pharma is developing a portfolio of phages targeting 3 of the most resistant and dangerous bacteria, which alone account for more than two thirds of hospital-acquired resistant infections: Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. The concept of precision phage therapy has been successfully applied in several dozen patients in the context of compassionate use, under the supervision of the French National Agency for the Safety of Medicines (ANSM). Headquartered in Nantes, Pherecydes Pharma has a team of around twenty experts from the pharmaceutical industry, biotechnology sector and academic research.
For more information, www.pherecydes-pharma.com
Contacts
Pherecydes Pharma
Thibaut du Fayet
Deputy CEO
investors@pherecydes-pharma.com
NewCap
Dusan Oresansky
Investor Relations
pherecydes@newcap.eu
T.: +33 1 44 71 94 92
NewCap
Arthur Rouillé
Media Relations
pherecydes@newcap.eu
T.: +33 1 44 71 00 15
This article was originally published here
Once a pressure injury has appeared, you need to take immediate action. Contact your health professional for all pressure injuries regardless of the stage. You may be referred to a wound care specialist to help heal your wound. Do not be embarrassed as you know pressure injuries develop for a variety of reasons, many of which do NOT include lack of care on your part. A pressure injury usually occurs due to combinations of reasons that can include self-care issues but also metabolic reasons … You need to be evaluated for treatment. If the wound is open, a treatment plan will be made for you to follow. If you see a change in pigmentation without an opening in the skin that you have dealt with previously, you may already know your plan. You will need to work with your healthcare professional if an area is repeating pressure injuries … read more
According to new research by investigators at the Mayo Clinic and Washington State University, e-bandages could be an effective alternative to antibiotics for managing wound infections. The findings are presented at ASM Microbe 2022, the annual meeting of the American Society for Microbiology … In a recent study conducted in mice, novel hydrogen peroxide producing bandages with electrical/chemical properties (electro-chemical bandages or e-bandages), under the control of wearable voltage devices, reduced methicillin-resistant Staphylococcus aureus (MRSA) biofilm bacteria present in the wound by 99 percent after 2 days of treatment … read more
13-Jun-2022 8:05 AM EDT, by Orpyx Medical Technologies Inc.
Newswise — CALGARY, AB, June 13, 2022 /PRNewswire/ — Orpyx® Medical Technologies Inc. (Orpyx), a digital health company focused on extending the health span of patients with diabetes, announced the launch of the Orpyx SI® Flex Sensory Insole system and Orpyx Remote Patient Monitoring (RPM) services. This remote monitoring program drives engagement and extends mobility for people living with diabetes by transforming patient care through real-world patient data, analytics, and coaching.

The Orpyx SI® Flex Sensory Insoles help reduce the risk of plantar complications by monitoring plantar pressure, adherence, step count, and temperature data for patients that need preventative care.
“The launch of Orpyx SI Flex is our next step in enabling Diabetes Healthspan Extension™,” stated Dr. Breanne Everett, CEO and co-founder of Orpyx. “In North America, one-third of people are living with diabetes or pre-diabetes which often requires juggling an overwhelming number of actions, measurements, and appointments. By taking a holistic approach through a digital care platform we can dramatically improve upon the current standard of care. We are starting with foot care through robust data generation via our sensory insoles, and supporting patient engagement through RPM and coaching.”
The Orpyx SI Flex Sensory Insole system is a wearable technology designed to help prevent plantar foot complications for at-risk patients with peripheral neuropathy. “It is like having a supercomputer on the bottom of each foot,” said Denis Brisson, Chief Operating Officer at Orpyx. “Plantar pressure is a leading cause of foot ulceration so by measuring sustained high pressure we can help eliminate the root cause of many of these wounds before they happen.”
Along with pressure, the system captures wear time to determine how compliant the patient is to their provider’s treatment plan, step count to help dose activity levels, and temperature the last line of defense to indicate when inflammation is present and tissue damage is occurring. The ultra-thin, prefabricated sensory insoles fit in most every-day footwear, making them practical and comfortable for a broad range of patients.
Orpyx is changing the conversation by initially focusing on preventing foot ulceration by generating the most robust set of real-world plantar data. Historically, the focus has been on how to treat patients’ foot ulcers after they have already formed, with disappointing results as 25% of people with diabetes will develop an ulcer during their lifetime. These ulcers have a detrimental impact on patients’ mobility often resulting in life-shortening events such as amputation which can cause a ripple effect of social isolation, mental health challenges, and in the most complex situations death. Most diabetic foot ulcers (DFUs) are preventable, so we are on a mission to help patients and providers achieve improved, sustainable outcomes.
“Healthcare systems cannot sustain the current clinical strain of managing the 34 million people with diabetes in the US and the resulting $176B in financial costs. One third of this cost is attributed to lower limb care which is why this is such important work,” continued Dan Hughes, Orpyx’s Chief Commercial Officer. “Orpyx SI Flex Sensory Insoles and our new RPM services were specifically developed to meet these challenges head-on. Our insoles will play a significant role in the diabetic care pathway enabling patients to take proactive steps to manage their diabetes while reducing the total cost of care for Commercial Payers and Integrated Delivery Networks across the United States. Ultimately, this technology drives alignment and better outcomes for patients, providers, payers, and physicians.”
Healthcare providers seeking additional information to help their patients prevent DFUs can learn more about the new Orpyx SI Flex Sensory Insoles at https://www.orpyx.com/orpyx-si-flex-sensory-insoles.
About Orpyx Medical Technologies Inc.
Founded in 2010, Orpyx® Medical Technologies Inc. (“Orpyx”) is a Calgary-based digital health company. We take a holistic approach to remote “anywhere” care, which includes the company’s proprietary, imperceptible sensory insole platform that detects pressure, temperature, gait, activity, and movement symmetry. Our comprehensive Orpyx SI® Sensory Insole system enables continuity of care between visits to provide optimal remote care for people living with diabetes or recovering from surgery. The patient’s data is uploaded to the Orpyx SI cloud-based dashboard, where accredited healthcare practitioners remotely monitor it. The remote monitoring team communicates directly with the patient between care visits to ensure ongoing continuity and quality of care.
Visit www.orpyx.com for more information.