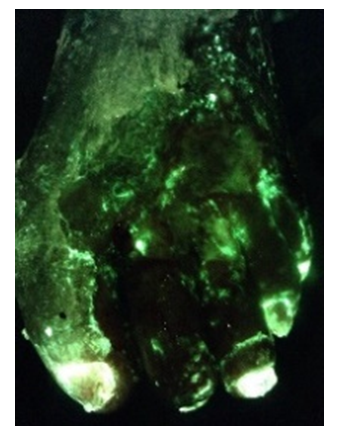
A submission on MolecuLight by Dr. Charles A. Andersen was one of the top scoring abstracts out of more than 200 submissions. This is the fifth consecutive SAWC meeting at which an abstract on improved patient care achieved through use of the MolecuLight i:X has received this honour.
The 5 clinical posters and 2 presentations featuring the MolecuLight i:X from SAWC Fall 2021 are as follows:
Poster #CR-005
12-Week RCT Evaluating Impact of Routine Fluorescence Imaging of Bacteria on DFU Healing Rates
Alisha Oropallo, MD¹, Scott Gawlik DPM¹, Dean Vayser, MD²
¹Northwell Comprehensive Wound Health Center and Hyperbarics, Lake Success NY,
²ILD Research Centre, San Diego, CA
Download poster
Poster #CR-006
Cleansing Techniques for Wound Hygiene: Which Are Most Effective?
Alisha Oropallo, MD1, Amit Rao MD1, Jai Joshi1
1Northwell Comprehensive Wound Health Center and Hyperbarics, Lake Success NY
Download poster
Poster #LR-025
Detection of bacterial fluorescence from in vivo wound biofilms using a point-of-care
fluorescence imaging device
Andrea J. Lopez1, Laura M. Jones2, Landrye Reynolds1, Rachel C. Diaz1, Isaiah K. George1, William Little1, Derek Fleming3,4, Anna D’souza2, Kendra Rumbaugh3, Allie Clinton Smith1, Monique Y. Rennie2
1Department of Honors Studies, Texas Tech University, Lubbock TX, USA; 2MolecuLight Inc. Toronto, ON Canada; 3Department of Surgery, Texas Tech University Health Sciences Center; 4Division of Clinical Microbiology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA
Download poster
Poster #CR-020
Are Semi-quantitative Clinical Cultures Inadequate? Comparison to Quantitative Analysis of 1053 Bacterial Isolates from 350 Wounds
Thomas E. Serena1, Phil Bowler2, Gregory Schultz3, Anna D’souza4, Monique Rennie4
1SerenaGroup Research Foundation, Cambridge MA USA; 2Phil Bowler Consulting, Warrington UK; 3Department of Obstetrics and Gynecology, University of Florida, FL, USA; 4MolecuLight Inc. Toronto
Download poster
Poster #PI-003
Guidelines for point-of-care fluorescence imaging for detection of wound bacterial burden based on Delphi consensus
Charles A. Andersen¹, Alisha R. Oropallo², Raymond Abdo³, Jenny Hurlow⁴, Martha R Kelso⁵, M. Mark Melin⁶ and Thomas E. Serena⁷
1Madigan Army Medical Center, Renton WA; 2. Zucker School of Medicine Hofstra/Northwell, Hempstead, NY; 3St. Louis Foot & Ankle LLC, St. Louis MO; 4Consultant Wound Care Practitioner, Memphis TN; 5Wound Care Plus LLC, Blue Springs MO; 6M Health Fairview, Edina MN; 7SerenaGroup Research Foundation, Cambridge MA
Download poster
Oral Presentation & Poster #PI-002
Diagnosis and Treatment of the Invasive Extension of Bacteria (Cellulitis) from Chronic Wounds Utilizing Point-of-Care Fluorescence Imaging
Charles Andersen¹, Katherine McLeod¹, Rowena Steffan¹
¹Vascular/Endovascular/Limb Preservation Surgery Service, Madigan Army Medical Center, Joint base Lewis-McChord, WA USA
Download poster
Podium Presentation
Innovation Spotlight: Shining a Light on Bold Ideas in Wound Care
Charles Andersen¹
¹Vascular/Endovascular/Limb Preservation Surgery Service, Madigan Army Medical Center, Joint base Lewis-McChord, WA USA
In additional to the clinical posters and presentations at SAWC (Symposium on Advanced Wound Care) Fall 2021, the recently launched MolecuLightDX will be available for demonstration in the MolecuLight booth #439 in the Exhibit Hall at Caesars Palace in Las Vegas, Nevada.
About MolecuLight Inc.
MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercially released devices, including the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, provide point-of-care handheld imaging devices for the global wound care market for the real-time detection of wounds containing elevated bacterial burden (when used with clinical signs and symptoms) and for digital wound measurement. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant, unmet needs including food safety, consumer cosmetics and other key industrial markets.
Download for Image:
SOURCE MolecuLight
Related Links
www.moleculight.com