a new class of molecules for treating biofilm infections

LSU Chemistry graduate student Leo Fontenot (left) conducting research under the guidance of Professor Mario Rivera (right).
BATON ROUGE- Chronic skin wounds are a growing global concern amongst aging populations and patients with severe burns and underlying health conditions such as diabetes. Commonly seen in healthcare environments and long-term care facilities, patients with chronic skin wounds suffer from persistent pain and potentially life-threatening infections.
Effective treatments for these wounds are often challenged by a biofilm, a community of bacterial cells entrenched in a self-produced matrix of extracellular DNA, proteins, and sugar molecules. Bacterial cells in biofilms are tolerant to the body’s immune response and most commercial antibiotics that normally kill free living, or planktonic, bacterial cells.
The antibiotic recalcitrance of biofilm bacteria is further complicated in biofilm infections with multidrug resistant, or MDR, bacteria. Therefore, successful treatment of MDR bacterial biofilm-associated infections require alternative treatment strategies.
LSU Professor and William A. Pryor Chair in Chemistry Mario Rivera recently received a five-year $3.69 million National Institutes of Health, or NIH, R01 grant to develop a new strategy to kill biofilm-embedded cells and combat biofilm infections caused by MDR bacteria, Pseudomonas aeruginosa and Acinetobacter baumannii.
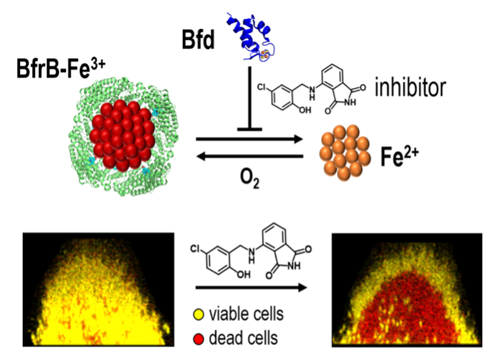
Small molecule inhibitors of the BfrB-Bfd complex disrupt bacterial iron homeostasis and kill biofilm embedded bacterial cells. [Source: https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00669. Further permission related to the material excerpted should be directed to the ACS.]
Bacterioferritin, or BfrB, is a spherical and hollow protein that can store thousands of iron atoms in its interior. Rivera and colleagues demonstrated that the mobilization of iron stored in BfrB is an essential process in bacterial cells, which requires BfrB to form a complex with Bfd. The scientists aim to disrupt the BfrB-Bfd protein-protein interaction.
They also discovered proof-of-concept small molecules that can inhibit the BfrB-Bfd complex and cause an irreversible accumulation of unusable iron in BfrB, which leads to iron deficiency, metabolic stress, and bacterial cell death in the biofilm. The proposed work supported by the new grant is directed at optimizing the proof-of-concept inhibitors of the BfrB-Bfd complex into drug lead molecules, that is, powerful inhibitors that kill bacterial cells in biofilms but also have desirable drug-like properties.
The funded research project involves a talented, multidisciplinary team of drug discovery collaborators from LSU and other institutions, including:
- Professor Mario Rivera (PI), Department of Chemistry, Louisiana State University
- Research Associate Professor Huili Yao (co-PI), Department of Chemistry, Louisiana State University
- LSU Chemistry postdoctoral researcher Anabel Soldano and graduate students Leo Fontenot, Nimesha Rajapaksha, Suliat Alli and Alexanndra Behm.
- Associate Professor Josephine Chandler (co-PI), Department of Molecular Biosciences, University of Kansas
- Dr. Scott Lovell (co-PI), Director of the Protein Structure Laboratory, University of Kansas
- Professor Richard Bunce (co-PI), Department of Chemistry, Oklahoma State University
- Professor Lisa Morici (co-PI), Tulane University School of Medicine
- Dr. Allen Reitz (co-PI), Fox Chase Chemical Diversity Center
If the proposed strategy proves successful, validation of the novel antibiotic target would lead to the development of an entirely new class of antibiotics for the treatment of biofilm-associated infections. To learn more about the Rivera research group and their work on iron homeostasis in bacteria as a potential target for antibiotic development, visit their group page.
Media Contact:
Gretchen Schneider
LSU Chemistry
gschne2@lsu.edu