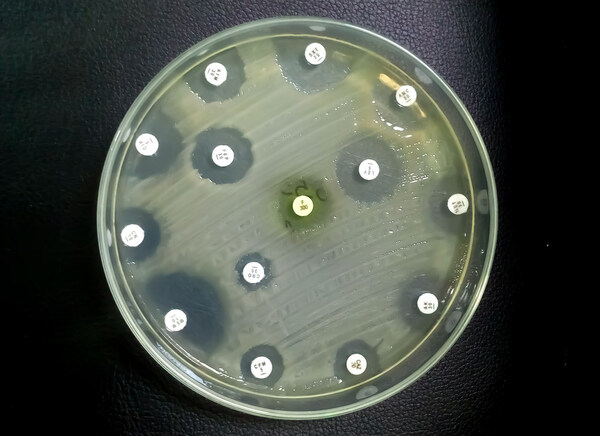
BOZEMAN, Mont., May 10, 2023 /PRNewswire/ – Microbion Corporation today announced that the company presented a poster focusing on pravibismane’s activity against diabetic foot ulcer infection pathogens at the 9th International Symposium on the Diabetic Foot that is currently ongoing from May 10th to 13th, 2023 at The Hague, Netherlands. The poster highlights pravibismane’s activity versus comparator antibiotics against pathogens isolated from diabetic foot infection (DFI) patients in an earlier Phase 1b clinical study. Poster Details:
Title: Broad-Spectrum, Potent Activity of Pravibismane Versus Comparators Against Diabetic Foot Ulcer Infection Patient Isolates Collected in a Phase 1b Study Presenter: Dr. Jeff Millard, CSO Poster Highlights:
"We are pleased that pravibismane demonstrated extremely potent MIC activity against clinical DFI isolates, which was in line with in vitro AST microbial pre-clinical studies," said Dr. Jeff Millard, CSO of Microbion Corp. "Diabetic foot infections are often infected by several different bacterial species concurrently, which may change over the chronicity of the wound, from predominantly aerobic to anaerobic. We believe pravibismane’s potent broad-spectrum activity is potentially a key treatment advantage since a single agent could eradicate both aerobic and anaerobic bacteria, thereby decreasing the need for multiple systemic therapies." Bacterial cultures for this study were grown from swabs collected at the wound bed at baseline visit and antimicrobial susceptibility testing (AST) was performed on isolated pathogens. Pathogen isolation and AST was performed at Investigational Health Management Associates (IHMA, IL), using the Clinical & Laboratory Standards Institute (CLSI) standard methods. Topical pravibismane has received QIDP and Fast Track drug designation from the US FDA for the adjunctive treatment of moderate and severe diabetic foot ulcer infections. Topical pravbismane is currently enrolling in a Phase 2 clinical study to further evaluate its safety and efficacy in subjects suffering from moderate infections associated with chronic diabetic foot ulcers. About Microbion
Microbion is a clinical-stage pharmaceutical company developing a new class of therapeutic compounds to improve the lives of patients with rare and serious diseases. Microbion’s lead drug candidate, pravibismane, is the first product in this new class and has multiple novel modes of action offering unique potential to address the unmet needs of chronic and severe health conditions. Topical/local pravibismane is in Phase 2 development for the treatment of chronic wounds and orthopedic infections. The Company is advancing inhaled pravibismane in Phase 1 clinical development for the treatment of chronic lung diseases, including non-tuberculous mycobacteria (NTM) and cystic fibrosis-related lung infections. Pravibismane has received backing from the Cystic Fibrosis Foundation, NIH, US DoD, and CARB-X with over $21 million in grants. The FDA has granted pravibismane with Orphan Drug, Fast Track, and QIDP designations. For more information visit: www.microbioncorp.com. Safe Harbor Statement
Certain of the statements made in this press release are forward-looking, such as those, among others, relating to the success of clinical development of pravibismane and preparation for potential commercialization. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: our ability to enroll patients in our clinical trials at the pace that we project; the size and growth of the potential markets for pravibismane or any future product candidates and our ability to serve those markets; our ability to obtain and maintain regulatory approval of pravibismane or any future product candidates; and our expectations regarding the potential safety, efficacy or clinical utility of pravibismane or any future product candidates. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Microbion Corporation disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. SOURCE Microbion Corporation |
Category: Press Releases
Orpyx Partners With Onduo to Offer Foot Ulcer Prevention Sensor as Part
of Virtual Diabetes Program
The addition of foot ulcer prevention to Onduo’s virtual diabetes program offering is significant. According to Singh et al., 25 percent of people with diabetes develop foot ulcers over their lifetime and today, one in five of those people experience complications that lead to amputation. Orpyx foot ulcer prevention technology will be available to select members of the Onduo community in 2019.
“Orpyx helps people with diabetes to prevent foot ulcers by providing insight that protects foot health and mobility and reduces the risk of complications that can lead to limb loss,” said Breanne Everett, CEO of Orpyx Medical Technologies Inc. “We are pleased to extend access to our foot sensor technology to the Onduo member community and to invite Orpyx U.S. patients to take advantage of Onduo services.”
Onduo integrates hardware and software to provide people with access to personalized, convenient diabetes care. People with diabetes are matched with lifestyle and clinical interventions, which for participating clients and select users will include wirelessly connected foot monitoring from Orpyx next year.
Orpyx FDA-cleared foot sensor technology is embedded in shoe insoles to monitor foot pressure and relay alerts to a smartphone or smartwatch when a person needs to take action to prevent foot injury. The technology is effective even for those with foot numbness, known as peripheral neuropathy. In the U.S. alone, almost one million diabetes-related foot ulcers are treated each year, costing upwards of $30,000 USD per ulcer with complications that can result in amputation.[2] Forty percent of people who experience one diabetes-related foot ulcer will have a second ulcer in the next year.[3] This number approaches 100 percent at 10 years.
“Managing diabetes is a 24/7 job and we want to make access to care and monitoring easier for members,” said Dr. Josh Riff, CEO of Onduo. “We are thrilled to partner with Orpyx to help keep members walking and living actively in our community.”
View source version on accesswire.com:
https://www.accesswire.com/530441/Orpyx-Partners-With-Onduo-to-Offer-Foot-Ulcer-Prevention-Sensor-as-Part-of-Virtual-Diabetes-Program
Corstrata Selected as a Venture Atlanta 2018 Startup Showcase Company
Corstrata announced today that it has been chosen as one of 34 Venture Atlanta Startup Showcase “companies to watch,” a group comprised of the most exciting early-stage businesses that are bringing big ideas to the next decade.
As a Venture Atlanta Startup Showcase Company, Corstrata will be spotlighted during a special networking event on October 16 where it will provide a “sneak peek” of its tech-enabled solutions and business plan and be given the opportunity to connect with investors, entrepreneurs and other technology leaders.
The 11th annual Venture Atlanta is the Southeast’s premier event for connecting technology innovation and investment capital and will take place October 16-17 in the heart of downtown Atlanta. With representation of over 140 funds and a roster of exciting speakers—including presentations from 33 of the region’s top rising star technology companies and keynotes from former NetSuite CEO Zach Nelson and Dave DeWalt, former CEO of FireEye, McAfee and Documentum—this year’s anticipated to be sold-out event is on track to be the largest and best yet.
“We are honored to be selected for Venture Atlanta’s 2018 Startup Showcase and the opportunity to showcase our innovative, mobile and digital wound care management solutions at this important event,” said Katherine Piette, Chief Executive Officer and Founder, Corstrata. “As an organization, our mission is to advance wound prevention and care across the spectrum of care by empowering home health agencies, hospice care organizations, skilled nursing facilities, inpatient rehab facilities and other healthcare providers to become wound care centers of excellence using the most effective wound care technology solutions available combined with on-demand, remote access to our board-certified wound care experts.”
AmpliPhi Biosciences Announces Updated Positive Clinical Results
for its Expanded Access Program
- 21 patients at 7 hospitals, with serious or life-threatening infections not responding to antibiotics, have now been treated with AB-SA01 (targeting S. aureus) or AB-PA01 (targeting P. aeruginosa) under AmpliPhi’s expanded access program
- Over 1,000 doses of bacteriophage product candidates, AB-SA01 or AB-PA01, have been administered as part of the expanded access program since mid-2017 and have been generally well tolerated, with no serious adverse events attributable to bacteriophage treatment
- 84% of patients achieved treatment success at the end of therapy
AmpliPhi Biosciences Corporation (NYSE American: APHB), a clinical-stage biotechnology company focused on precisely targeted bacteriophage therapeutics for antibiotic-resistant infections, today announced updated topline clinical results for its ongoing single-patient expanded access program. 84% of patients achieved treatment success (physician’s assessment) at the end of therapy, defined as complete resolution or significant improvement of baseline signs and symptoms.
AmpliPhi has now provided its investigational bacteriophage therapeutics for a total of 21 patients, at 7 hospitals, with serious or life-threatening infections not responding to antibiotic therapy. These patients were treated with AB-SA01 or AB-PA01 under single-patient expanded access programs in the U.S. (Emergency INDs per the U.S. Food and Drug Administration) or Australia (Special Access Scheme per the Australian Therapeutic Goods Administration). The following analysis updates the data previously announced by the company on January 3, 2018 … read more
KMT Medical Announces Ownership of US-Based Twenty Second
Company and ABC Medical Holdings, Inc.
TAMPA, Fla., Sept. 14, 2018 /PRNewswire/ — KMT Medical Incorporated (KMT Medical), an international group of companies providing healthcare services to consumers, announces its ownership of the US-based holding company, Twenty Second Company and its operating subsidiary ABC Medical Holdings, Inc (ABC Medical). These companies will join their European Service Business counterparts, operating under KMT Medical.
ABC Medical is one of the nation’s leading and fastest growing homecare companies for urology. The ABC Medical team is the leading provider in the industry committed to adaptive sports sponsoring over 100 events annually. ABC Medical also provides ostomy, incontinence and wound care services. This company operates under the philosophy of providing legendary customer service and honoring clinical and patient choice.
Announced in 2017, KMT Medical is a wholly owned subsidiary of The Firm of John Dickinson Schneider Inc. (JDS Inc). “KMT Medical companies share the same patient-focused mission of the parent company,” says V. George Maliekel, President and Chief Executive Officer of JDS Inc, “to make life more rewarding and dignified for people who use our products and services, and the same vision, to grow and prosper as an independent company and in the process to become better human beings.” These companies operate under and adhere to a set of policies that include respecting clinical decisions, honoring patient choice in matters of product selection, and collaborating with payers and suppliers to provide high quality products and personalized services to consumers.
Healogics, Inc. Names Michael Fogarty as New Chief Operating Officer
JACKSONVILLE, Fla.–(BUSINESS WIRE)–Healogics®, the nation’s wound expert, today announced that it has appointed Michael Fogarty to serve as Chief Operating Officer. He will be replacing Greg Martin who will be retiring at the end of this year. In this role, Fogarty will be responsible for providing management and operational oversight for all lines of business, including the outpatient Wound Care Centers® and other wound care programs in Healogics’ portfolio of services. Additionally, he will evaluate the potential integration of process re-engineering initiatives to ensure Healogics is addressing customer needs while optimizing an efficient operations function. Fogarty will report directly to the Chief Executive Officer, David Bassin.
We are excited to add Mike’s extensive process improvement, payer and healthcare experience to our organization,” said Bassin. “I am confident that with his background, skills and experience, we will be able to take our organizational service and value to the next level.”
Fogarty is an accomplished leader with over 30 years of experience in the healthcare and financial services industries, including his most recent position with UnitedHealth Group® as the Senior Vice President … read more
Kekst Works Avista’s Wound Care Deal
Kekst & Co is handling Avista Capital Markets private equity firm’s merger of a “blank check company” with Organogenesis Inc. advanced wound care and sports medicine operation.
ACM has agreed to invest $92M in Canton, MA-based Organogenesis, which is expected to trade on the NASDAQ with an enterprise value in the $675M range when the deal closes by the end of the year.
Organogenesis has more than 600 employees involved in the development, manufacture and commercialization of products in the regenerative medicine sector.
Tom Dean, CEO of Avista Healthcare Public Acquisition Corp., said Organogenesis is “well-positioned to benefit from secular tailwinds driving the advance would care, surgical and sports medicine sectors.” … news release from O’Dwyer’s
Lone Peak Biologics is Excited to Announce its Newest Groundbreaking
Regenerative Product: AmnioShot™
Amniotic fluid is used in many orthopedic diseases that are resistant to standard techniques. AmnioShot™ is derived from the amniotic fluid of donated birth tissues and leverages the healing properties of amniotic fluid for clinical applications.
Lone Peak Biologics is thrilled to announce the latest addition to its industry-leading line of human biologics products: AmnioShot™. AmnioShot™ leverages the healing properties of amniotic fluid for clinical applications, and has proteins that are known to promote healing, and reduce or inhibit adhesion and infection in a number of scenarios, some of which include:
- Wounds
- Osteo-Arthritis, Tendinitis
- Osteogenesis
Amniotic fluid has been used in many orthopedic diseases that are resistant to standard techniques, and it is being explored extensively to develop innovative therapies for other conditions. Allogeneic amniotic fluid has been used in Achilles tendinosis and plantar fasciosis by incorporating the amniotic fluid allograft as a suspension tissue scaffold or matrix to assist in tendon and fascia repair.
Furthermore, amniotic fluid is known to have low immunogenicity and contains carbohydrates, proteins, lipids, lactate, pyruvate, electrolytes, enzymes, and hormones. Additionally, amniotic fluid contains anti-inflammatory and osteo-promotive agents such as hyaluronic acid, epidermal growth factors (EGF)-angiogenic, transforming growth factors (TGFa and TGFb), and insulin-like growth factors (IGF).
Moreover, it can be used to support re-epithelialization, aid joints and connective tissues, and as a complement to bone grafts for spine fusions and other bone-healing procedures. Studies on the potential of products like these in regenerative medicine have yielded numerous results, and studies demonstrating the use of amniotic products have been largely successful.
Extraction of Amniotic Fluid … read more
SomaGenics Awarded Multi-Year Funding for Hepatitis Delta Virus Therapeutic Program
SANTA CRUZ, Calif., July 30, 2018 /PRNewswire/ — SomaGenics, Inc. announces the award of a three-year, $2.9 million NIH grant in support of its Hepatitis Delta Virus (HDV) therapeutic program under Principal Investigator and SomaGenics CEO Brian H. Johnston, Ph.D. This Phase II grant, from the NIH’s Small Business Innovation Research (SBIR) program, continues the development of SomaGenics’ novel RNA interference (RNAi)-based approach under a Phase I SBIR grant and will fund late-stage preclinical studies and preparations for clinical trials.
HDV infection, which requires concurrent or prior infection with the hepatitis B virus, results in the most severe form of viral hepatitis, and no HDV-specific therapy exists. Chronic HDV has a 20% mortality rate and its incidence is rising globally. The establishment of U.S.–based Hepatitis Delta Connect (hepconnect.org), a public outreach program, highlights recent efforts to increase patient and physician awareness about the pressing need for HDV screening and treatment.
SomaGenics’ HDV therapeutic is a novel treatment modality simultaneously targeting the virus at multiple stages of its life cycle using the Company’s proprietary synthetic short hairpin RNA (sshRNA®) technology. “Current clinical treatments suffer from multiple problems including limited efficacy, high relapse rate and toxicity,” according to Anne Dallas, Ph.D., Principal Scientist.
To date, the Company has demonstrated efficacy of its sshRNA® HDV therapeutic in cell culture models and will use the new NIH funding to support efficacy studies in animal models as well as to optimize the Company’s novel delivery platform. “Our combination, multi-target approach reduces the likelihood of treatment resistance and targets non-host entities, lowering the chance of toxicity. We are excited that SomaGenics’ therapeutic may have the potential to cure HDV patients,” explains Dr. Dallas.
Somagenics’ sshRNAs® are highly potent RNAi triggers, with IC50’s in the low picomolar range. sshRNAs® have distinct advantages over the more familiar siRNAs, including the fact that they consist of single chemical entities, simplifying their production and purification, and their lack of off-target effects from “passenger” strand retention. sshRNAs® are suitable for use in many indications in addition to HDV, with therapeutics currently in development for chronic wound healing including diabetic foot ulcers.
SomaGenics, Inc. is a privately held company with offices and laboratories located in Santa Cruz, California. The Company specializes in developing novel RNA-centered approaches to address unmet life science research and medical needs. Core competencies include RNA molecules as therapeutic agents, drug targets and biomarkers as well as the development of innovative kits for RNA analysis.
For information on SomaGenics’ HDV program or the sshRNA® platform, please contact Anne Scholz, VP Business Development, 831-426-7700 x20, 199087@email4pr.com
SOURCE SomaGenics, Inc.
Osiris Announces Enrollment of Patients in a Clinical Trial Evaluating
GrafixPL PRIME™ in the Treatment of Chronic Venous Leg Ulcers
COLUMBIA, Md., July 11, 2018 (GLOBE NEWSWIRE) — Osiris Therapeutics, Inc. (OTC Pink:OSIR), a regenerative medicine company focused on developing and marketing products for wound care, orthopedics, and sports medicine, announced the initiation of its “Multicenter, Prospective, Randomized, Open-Label Study with a Crossover Extension Option to Evaluate the Safety and Efficacy of GrafixPL PRIME™ in the Treatment of Chronic Venous Leg Ulcers.”
This study is expected to enroll up to 200 patients in approximately 30 clinical sites. Patients will be randomized 1:1 to receive GrafixPL PRIME plus standard compression therapy (SOC) versus SOC alone in patients with chronic venous leg ulcers (VLUs). The study objective is to evaluate the safety and efficacy of weekly applications of GrafixPL PRIME plus SOC versus SOC alone for chronic VLUs with a size between 1 cm2 and 25 cm2. Patients in the SOC alone group whose ulcers do not close will be offered GrafixPL PRIME adjunct to SOC in a crossover extension treatment phase of up to 12 treatments … read more
Gel-E wins FDA OTC nod for gel-e Flex flowable hemostat
Early stage wound care company Gel-e said today it won FDA 510(k) clearance for its gel-e Flex as an over-the-counter flowable hemostat.
The College Park, Md.-based company said the clearance expands the labeling for the local management of lacerations and minor bleeding, and said the products were specifically designed to be usable by both trained professionals and patients.
2018 Innovations in Neuro Therapies, Wound Management
Injectable Bandages, Biosensors, Skin Electrocardiograms, and Medical Cables – ResearchAndMarkets.com
DUBLIN–(BUSINESS WIRE)–The “Innovations in Neuro Therapies, Wound Management, Injectable Bandages, Biosensors, Skin Electrocardiograms, and Medical Cables” report has been added to ResearchAndMarkets.com’s offering.
The latest edition of Medical Device TOE profiles a wide diversity of innovations impacting the healthcare industry.
This issue identifies and describes early-stage innovations such as neuromodulation therapy to manage post-traumatic stress disorder (PTSD), implantable sensors for continuous medical monitoring, and bodyworn monitoring devices for mobile cardiac monitoring.
Mature technologies that have been, or are nearing, commercialization, such as drug-coated balloons for peripheral artery disease, and a novel delivery vehicle for timed release of vaccines are also discussed.
Key Topics Covered:
- Neuromodulation Therapy for Traumatic Brain Injury
- Pneumatic Compression Therapies for Lymphedema
- Urinary Bladder Matrix for Wound Management & Soft Tissue Repair
- Wearable Headphone to Completely Clear-out Earwax Buildup
- Connected Device for Analysis of Wheezing Trend
- Injectable Biosensors to Stream Medical Data
- Ultra-thin Wearable Electrocardiogram Device
- A Novel Biomaterial to Design Cancer Vaccines
- Novel Drug-coated Balloon for Peripheral Artery Disease Treatment
- Ultrathin Insulation for Medical Cables
- Database of Key Industry Participants
For more information about this report visit https://www.researchandmarkets.com/research/nb5dbk/2018_innovations?w=4
Contacts
ResearchAndMarkets.com
Laura Wood, Senior Manager
press@researchandmarkets.com
For E.S.T Office Hours Call 1-917-300-0470
For U.S./CAN Toll Free Call 1-800-526-8630
For GMT Office Hours Call +353-1-416-8900
Related Topics: Biotechnology, Infusions and Injectables , Wound Care
View source version on businesswire.com:https://www.businesswire.com/news/home/20180628006367/en/
Healogics Releases Software to Improve Chronic Wound Care Experience
JACKSONVILLE, Fla.–(BUSINESS WIRE)–Healogics®, the wound healing experts, today announced the launch of two new applications that support efforts to improve the patient experience and save time for clinicians and physicians, all while increasing the quality and consistency of patient care. Clinical OptimizationSM and Decision SupportSM, applications on Healogics’WoundSuiteSM platform, enable the critical connection between people living with chronic wounds and their multi-disciplinary healthcare team for collaborative, evidence-based, patient-centered care.
“Over the past year, Healogics has been working tirelessly to ensure that the care teams at our Wound Care Centers® have access to the best software available to help more people heal. We are excited about the launch of these new applications, and their ability to support more in-depth documentation, accurate wound measurements and, most importantly, better patient outcomes,” said David Bassin, Healogics CEO.
Healogics Clinical Optimization provides clinicians and physicians with patient-focused insights starting with the daily team huddle. Additionally, this application supports them throughout the Healogics Patient Care ProcessSM, a six-sigma lean productivity process used in each Wound Care Center. Clinical Optimization provides a one-click patient summary that eliminates all of the arduous and time-consuming paper processes built around EMRs. By concisely presenting the essential patient information, physicians can now go through medical surveillance, a process designed to monitor patient healing, with case managers before ever walking into a patient’s room. This allows more time for meaningful patient interaction.
MediWound Completes Enrollment in NexoBrid® U.S. Phase 3 DETECT Study
YAVNE, Israel, June 11, 2018 (GLOBE NEWSWIRE) — MediWound Ltd. (Nasdaq:MDWD), a fully-integrated biopharmaceutical company bringing innovative therapies to address unmet needs in severe burn and wound management, today announced it has completed enrollment in NexoBrid®U.S. Phase 3 DETECT study. Top-line acute data are currently expected around year end 2018.
“We are happy to achieve this important milestone of completing the enrollment in NexoBrid Phase 3 study, which is one of the most comprehensive randomized controlled studies ever conducted in burn care, and we believe it will support our Biological License Application (BLA) submission to the FDA,” said Gal Cohen, president and chief executive officer of MediWound. “Prior studies of NexoBrid have shown positive results and we eagerly await our top-line acute data. Subject to a successful study outcome, we plan to meet with the FDA to discuss the BLA submission plan. We warmly thank our Principal Investigators, their teams and everyone involved in the study for their commitment and dedication in an effort to advance burn care.”
Organogenesis Inc. Announces Support of American Diabetes Association
Organogenesis will support initiatives to educate about diabetic foot ulcers, the leading cause of diabetes-related amputations
CANTON, Mass., June 21, 2018 /PRNewswire/ — Organogenesis Inc. – a leading regenerative medicine company committed to empowering healing – today announced it is a proud supporter of the American Diabetes Association (ADA). Organogenesis will support the production of an ADA scientific compendium focused on the latest scientific evidence related to the treatment of diabetic foot ulcers (DFUs), the leading cause of diabetes-related amputations in the United States.
Organogenesis will also host a corporate symposium, “Innovations in Diabetic Wound Healing,” at the ADA’s 78thScientific Sessions, held June 22-26 in Orlando, FL, and exhibit during the meeting trade show at Booth #616.
“As a global regenerative medicine company driven by a shared mission to empower healing, we work to provide opportunities for patients and health care professionals to learn more about state-of-the-art approaches to treat diabetic foot ulcers,” said Gary S. Gillheeney, Sr., President and CEO of Organogenesis. “We are happy to support the ADA and its work to help Americans living with diabetes.”
Breakthrough Randomized Controlled Trial Demonstrating TWO2
Efficacy in Healing Diabetic Foot Ulcers Unveiled at the American Diabetes Association 78th Scientific Sessions Conference
OCEANSIDE, Calif., June 26, 2018 /PRNewswire/ — AOTI Inc. announced today that initial results from its recently concluded Randomized Controlled Trial (RCT) demonstrating the efficacy of its patented multi-modality Topical Wound Oxygen (TWO2) homecare therapy in healing Diabetic Foot Ulcers were presented as a prestigious Late Breaking Abstract this weekend at the American Diabetes Association (ADA) 78th Scientific Sessions conference in Orlando, Florida.
The ADA estimates that Diabetes costs the USA$327 billion annually with a large portion of this cost related to treating comorbidities such as Diabetic Foot Ulcers (DFU)1. Non-healing DFU lead to increased mortality, morbidity & health economic burden as well as decreased QOL for the sufferers.
The study was conducted at seventeen Diabetic Foot Centers of Excellence spread across the USA and Europe, with a “who’s who” of expert Opinion Leaders as its investigators. The authors of the abstract highlighted the robustness of the study protocol that was of the highest scientific level ever seen in DFU studies, being not only conducted multi-center and multi-nationally, but being also double-blinded and placebo controlled. Additionally, the study protocol included the unprecedented step of a run-in of gold standard-of-care (SOC) for all subjects meeting enrollment eligibility criteria, ensuring that only those that truly failed to heal with SOC alone would be randomized into the active phase of the study.
full press release PR Newswire
Advanced Wound Care Management Market Estimated to Expand
at a 6.3% CAGR Through 2025
Albany, NY — (SBWIRE) — 05/26/2018 — Global Advanced Wound Care Management Market: Snapshot
The global market for advanced wound care management is poised for healthy growth in the upcoming years. Rising incidence of chronic wounds that require timely medical care before transforming into infection or other complications is fuelling the advanced wound care management market. Increasing prevalence of diabetic ulcers due to increasing incidence of diabetes I and II mellitus especially in developed countries is stoking demand for advanced wound care products. Worldwide, growing geriatric population susceptible to falls and injuries that can convert into chronic wounds is aiding the advanced wound care management market.
Request A Sample … read more
American Biotech Labs, LLC Receives Awards
and New Approvals
AMERICAN FORK, UT, May 31, 2018 /PRNewswire/ – American Biotech Labs, LLC (ABL) has received three new awards including: Total Health Magazine’s “Award of Excellence” and two Best of State awards for “Health and Wellness” and “Medical Manufacturing“. ABL is a health and wellness biotech company that continues to innovate products for the medical and health industries and has received a number of FDA clearances for wound dressing gel products, including antibacterial products for both wound dressing and wound cleansing.
ABL has now engineered the power of silver into antimicrobial moisturizing lotions and creams. Said Keith Moeller, CEO of American Biotech Labs, “Creating lotions and creams that are made with the antimicrobial advanced healing power of silver is a pretty daunting task, but being able to keep the moisturizers at greater than 99% organic, shows the true excellence of what we have created”.
Health Canada has already approved for sales and distribution these new antibacterial lotion and cream products and distribution is anticipated to begin in the Canadian markets within the next few months. In the US markets, ABL has begun distribution of these powerful lotion and cream cosmetic products under the Silver Biotics brand of products.
NPUAP Announces Release of Educational Tools for Wound Care Providers
PRESS CONTACT: Liz Posner, lposner@douglasgould.com, 646-214-0514, ext. 3
Washington, D.C. – June 15, 2017 – The National Pressure Ulcer Advisory Panel (NPUAP) announced bundle pricing of the new digital slide sets, designed to assist in the continuing education of health care professionals to prevent and treat pressure injuries.
Wound care providers working in acute care, post-acute care, home care agencies, and schools of nursing will benefit from the use of the slide sets. They include the 2016 Pressure Injury Staging System Teaching Slide Set and the Prevention of Pressure Injuries Slide Set, both available for $75 each, and the Treatment of Pressure Injuries Slide Set, available for $90. Buy all 3 for $199- a savings of over $40! All are instantly downloadable in PDF format at the NPUAP online store.
The Pressure Injury Staging System Teaching Slide Set is the result of the NPUAP 2016 Staging Consensus Conference. The Prevention of Pressure Injuries Slide Set and the Treatment of Pressure Injuries Slide Setare based on the current International Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline.
“The primary mission of the NPUAP is to improve patient outcomes in pressure injury prevention and treatment,” said NPUAP President Mary Litchford, PhD, RDN, LDN. “By incorporating the latest scientific evidence into the educational slide sets for teaching health care providers, the NPUAP is supporting its patient-focused mission.”
Wound care providers can find a number of other resources available on the NPUAP online store, including webinar recordings, books, and high-resolution photographs of sample wounds for educational purposes.
###
About the National Pressure Ulcer Advisory Panel
The National Pressure Ulcer Advisory Panel (NPUAP) is the nation’s leading scientific expert on pressure injury prevention and treatment. Our goal is to insure improved patient health and to advance public policy, education, and research.
WoundRounds® Launches New Mobile App for Wound Management
Schaumburg, Illinois (For Release Aug, 29, 2016) –WoundRounds®, the leading wound management solution, has announced their comprehensive wound documentation system is now available as a mobile app. The mobile app is available for WoundRounds clients on the Apple App Store.
“Adoption of mobile devices and applications has accelerated in recent years,” says Mike Diamond, CEO of Telemedicine Solutions, makers of WoundRounds. “The WoundRounds mobile app allows healthcare facilities to expand use of the WoundRounds solution to Apple mobile devices, leading to enhanced clinical outcomes and staff efficiencies.”
Data security is a growing concern for healthcare workers’ mobile devices, in light of a recent health system’s fines for HIPAA violations resulting from the theft of a staff member’s mobile phone. Diamond states, “The WoundRounds app works with the facility’s mobile device management (MDM) tools and meets strict security requirements to safeguard protected health information … read more
Clinical Evidence Presented at SAWC on Application of AMNIOX Medical Products
Four Posters Demonstrate the Clinical Benefits of NEOX® Wound Allograft Presented at SAWC
MIAMI–(BUSINESS WIRE)–AMNIOX Medical, Inc., a TissueTech, Inc. company, highlighted four clinical posters demonstrating the effectiveness of NEOX® Wound Allograft at the Symposium on Advanced Wound Care (SAWC) in April.
The presentations illustrate applications of NEOX Wound Allograft in combination with other therapies, and among a range of severe wounds that are resistant to current standard of care or are under served.
“The clinical effectiveness and value of NEOX continues to be reinforced in these studies, as well as opportunities to combine different approaches to advanced wound care,” said Adrian Roji, Chief Commercial Officer of TissueTech. “The authors found that patients who received NEOX experienced closure of hard-to-heal wounds, further highlighting the unique properties of cryo-preserved umbilical cord tissue.”
The studies presented at SAWC included:
- Treatment of Post-Operative Left Temporal Wound with Umbilical Cord Based Particulate and Negative Pressure Wound Therapy, Timothy Plackett, DO
- Use of Hydrated Shelf Stable Umbilical Cord Allograft for the Treatment of Chronic Wounds, Allen Raphael, DPM
- Treatment of Close-Range Gun Shot Wound with Umbilical Cord Based Particulate (pUC) and Negative Pressure Wound Therapy Timothy Plackett, DO
- Treatment of Invasive Carcinoma Wound with Hyperbaric Oxygen and Cryopreserved Umbilical Cord, Luis Fernandez, MD
AMNIOX parent TissueTech pioneered the commercialization and clinical application of human umbilical cord and amniotic membrane to promote healing. AMNIOX utilizes its proprietary CryoTek® process, a cryopreservation technology, to preserve the biological and structural integrity of these tissues more effectively than other available technologies. Since the company’s inception, clinicians have performed more than 300,000 human transplants of its products and published more than 300 peer-reviewed studies supporting its technology platform.
About AMNIOX Medical, Inc.
Founded in 2011 to serve the orthopedic and wound care markets, AMNIOX Medical is dedicated to developing and marketing products processed from umbilical cord and amniotic membrane utilizing its proprietary CryoTek technology. AMNIOX Medical procures its tissue through elective donation following healthy live birth via Cesarean section. Thorough donor screening is performed to ensure safety of its products. For additional information, please visit http://www.AMNIOXmedical.com.
About TissueTech, Inc.
TissueTech, Inc., the parent company of AMNIOX Medical, Inc. and BioTissue, Inc., pioneered the development and clinical application of amniotic tissue-based products. AMNIOX Medical develops and markets products for use in the musculoskeletal and wound care markets; BioTissue develops and markets products for the ophthalmology and optometry markets. The National Institutes of Health (NIH) has supported TissueTech’s research with more than 30 continuous years of research grants. Since the company’s inception, clinicians have performed more than 300,000 human implants of the company’s products and published more than 300 peer-reviewed studies supporting its technology platform. The Company’s first product, AmnioGraft®, is the only tissue graft designated by the FDA as homologous for promoting ophthalmic wound healing.
TUV Nord Awards ISO 13485 Certification to Keneric Healthcare
for the Design/Development, Manufacturing and Distribution of Wound Care Products
ALLEN, Texas, April 26, 2018 /PRNewswire/ — Keneric Healthcare’s ISO certification award represents its ability to design, implement, and successfully manage an effective quality management system that meets the requirements of DIN EN ISO 13485: 2012 / EN ISO 13485: 2012 +AC: 2012 – Medical devices – Quality management systems – Requirements for regulatory purposes. The scope of the ISO certification supports the Design/Development, Manufacturing and Distribution of Wound Care Products.
Keneric Healthcare, a global medical device manufacturer, recognized for the development and commercialization of innovative products that improve patient care, increase clinician efficiency and streamline facility expenditures. Product portfolio includes RTD™ Wound Dressing, an FDA cleared, polyurethane silver foam dressing that consistently demonstrates positive clinical outcomes for a variety of wound types; and PurePurge™ Bed bath System, an easy to use rinse-free patient bathing product offering healthcare professionals an ideal and cost-effective solution for bathing patients who are unable to take a traditional bath or shower.
Edixomed: Breakthrough Wound Care Technology
With Potential to Strike Back Against the Threat of Killer Superbugs
A simple patch which cleverly harnesses part of the body’s own natural repair system – nitric oxide – could help in the fight against killer superbugs and antibiotic resistance according to recently published studies.
Millions of people are at risk of dying from infections every day, many of which can no longer be treated by even the strongest antibiotics.
Now, in two recently published studies, a breakthrough wound care system, created by the UK firm Edixomed, has been shown to kill a range of antibiotic-resistant bacteria including MRSA and E. Coli, both of which have the potential to be fatal for many people.
The discovery could pave the way for these easy-to-use patches to be available in hospitals across the NHS to dress wounds to prevent the growth of bacteria, and tackle established infections.
“Bacterial infections resistant to all currently available antibiotics are expected to kill over 10 million people a year by 2050. The threat is very real and of international concern; but with this technology, we have a novel, viable and innovative solution with which to strike back. Wound care is just the first of many potential applications,” said Professor Art Tucker, St Bartholomew’s Hospital, London. He added, “Importantly, nitric oxide acts against multiple targets in bacteria to kill them, hence there is a very unlikely chance of bacteria developing resistance any time in the future.”
In addition, Edixomed’s breakthrough, the EDX110 wound care system, is able to deliver nitric oxide in a sustained way to give the wound or ulcer the best chance of healing. As part of the natural healing process the body normally produces nitric oxide and uses it to signal for increased blood flow and to fight infection. Edixomed’s technology effectively “supercharges” the body’s own natural healing processes.
In fact, recently published clinical research in diabetic foot ulcers, including infected ulcers, showed that the EDX110 patch achieved the same healing in 4 weeks as the standard-of-care approaches currently used in UK hospitals achieved at 12 weeks. The standard-of-care patients were also significantly more likely to be hospitalised due to complications with their foot ulcer.
“Diabetic foot ulcers are notoriously hard-to-heal and are the leading cause of diabetes-related amputations in the UK. The recently published findings provide an essential step forward in developing solutions for the effective management of these chronic wounds,” said Professor Michael Edmonds, Principal Investigator of the pivotal diabetic foot ulcer study, King’s College Hospital, London. He added, “Reducing infection and accelerating healing could significantly contribute to a reduction in the number of avoidable amputations. EDX110 represents a major step forward in best practice care.”
In severe cases, infection of a foot ulcer in a patient with diabetes can result in lower limb amputation or worse e.g. complications such as sepsis, multi-organ failure and death.
Facts:
- The NHS carries out more than 7,300 amputations each year in the UK as a result of diabetes, 80% of these are due to foot ulcers.[4] The resulting drain on healthcare resources is enormous, with an annual cost of £1 billion to NHS England alone.[5]
- At least 700,000 people die globally from drug-resistant infections every year – 5,000 of them in the UK.[6],[7]
- There have been no new classes of antibiotics approved since the 1980s and the Chief Medical Officer, Professor Dame Sally Davies warned in 2017 that resistance to antibiotics “poses a catastrophic threat”.[8]
Key findings of the two recent studies investigating EDX110, a revolutionary new wound care system:
- Laboratory tests have shown that EDX110 can kill all viable organisms for several deadly antibiotic-resistant infections including MRSA, Pseudomonas and E. Coli.[3]
- Laboratory tests have shown that EDX110 effectively prevented and treated multi-drug resistant bacteria biofilms. Biofilms are colonies of bacteria that protect themselves from the body’s immune system and actions of antibiotics.[3]
- EDX110 completely healed more ulcers compared with standard-of-care (ulcers completely healed: 49% vs. 30%).[2]
- EDX110 reduced diabetic foot ulcer size by almost double the amount of standard-of-care (median percentage area reduction: 89% vs. 47%).[2]
About Edixomed
Edixomed is a biopharmaceutical company commercialising next generation and clinically-proven technologies from its nitric oxide platform. Using its patented scientific approach, the company’s technologies have the potential to tackle major global health challenges in wound care, dermatology and infection control. The core technology’s unique feature is its ability to replenish or supplement the body’s own supply of nitric oxide that is critical for sustaining healthy skin and organs. Nitric oxide is depleted or absent in many diseases and thus, the body’s normal healing and regenerative processes are damaged. Restoring that essential element is at the heart of Edixomed’s approach to innovative healthcare.
About EDX110
EDX110 is a revolutionary, easy-to-use, two-part wound care system, driven by Edixomed’s core sustained-release nitric oxide delivery technology. EDX110 provides a protective and cushioning layer that uniquely absorbs fluid while providing a moist environment and generating nitric oxide. The role of nitric oxide in ulcer healing involves three recognised elements: vascular, as nitric oxide influences the widening of blood vessels (vasodilation) and stimulates the growth of new blood vessels (angiogenesis);[9],[10] inflammatory, as nitric oxide influences the body’s immune response;[11] and antimicrobial, as nitric oxide demonstrates potent, broad spectrum antimicrobial activity.
EDX110 is not yet an approved product, Edixomed are pursuing an active programme to develop applications of their core technology in multiple wound care indications and a number of additional areas. These areas include: surgical wound care, venous leg ulcers, pressure ulcers, burns, septic shock, transdermal drug delivery, ventilator-associated pneumonia, cystic fibrosis, and various applications connected to antimicrobial resistance.
About nitric oxide
The discovery that a simple gas, nitric oxide, could play such an important role in the human body led to three scientists being awarded the Nobel Prize for medicine in 1998. The pioneering work demonstrated that the normal function of nitric oxide is to control blood flow in the small vessels in the skin and prevent the skin from being infected with dangerous organisms. Nitric oxide is also generated whenever the skin is injured or damaged and plays a crucial part in the normal skin healing process. However, in certain conditions, such as diabetes, the normal production of nitric oxide can be put at risk and the skin loses the essential ingredient it needs to repair itself. The result is a chronic, poorly healing wound, highly prone to infection and a major cause of concern to patients and doctors. Replenishing the missing nitric oxide in such a way as to mimic the skin’s natural production is no easy task and it has eluded many of the world’s leading scientists for the past two decades. Edixomed has succeeded in achieving this goal and has demonstrated the performance of its technology in a pivotal clinical trial.
You can also visit our website at: http://www.edixomed.com
Aroa Biosurgery and Hydrofera Team up in US
MANCHESTER, Conn. & AUCKLAND, New Zealand–(BUSINESS WIRE)–New Zealand biomedical company Aroa Biosurgery and United States medical products company Hydrofera are launching Appulse in North America.
The two companies have recently bought back their wound care businesses from Hollister Inc.
Brian Ward, CEO of Aroa Biosurgery, says the company’s Endoform wound care technology and Hydrofera Blue are complementary.
“We have both been successfully represented by Hollister, but with Hollister’s decision to divest portions of its wound care business back to the technology developers, it gives us the opportunity to now grow the businesses ourselves.”
Tom Drury, CEO of Hydrofera, says: “On June 1 we will launch Appulse and most of the Hollister sales team for Endoform and Hydrofera Blue will continue to represent both products.
“This will help ensure the same level of service to patients, clinicians and distribution partners.”
Endoform is a proprietary extracellular matrix (ECM) biomaterial, which contains a collagen scaffold and important secondary molecules to support healing.
New Clinical Data for Covalon’s ColActive®
Line of Patented Advanced Wound Care Products to be Presented at SAWC Spring
MISSISSAUGA, Ontario–(BUSINESS WIRE)–Covalon Technologies Ltd. (the “Company” or “Covalon”) (TSXV: COV), an advanced medical technologies company, today announced that two clinical case studies demonstrating the clinical effectiveness of ColActive® Plus and ColActive® Transfer biological matrix products will be presented at the 2018 Symposium on Advanced Wound Care Spring (“SAWC Spring”) in Charlotte, North Carolina. In addition to the posters, Covalon will also be showcasing its full line of advanced wound care dressings in the symposium’s exhibition hall during the three-day event.
“We are very pleased with the results of these two clinical case studies and believe that they show the advantage that the patented ColActive technology can add to a wound care clinician’s arsenal,” said Brian Pedlar, Chief Executive Office of Covalon.
Dr. Jeffrey Lehrman, DPM, FASPS, MAPWCA will present a clinical poster entitled A New Approach in the Management of Chronic Diabetic Foot Ulcers: A Report on the Use of a Collagen Wound Contact Layer with Negative Pressure Wound Therapy during the SAWC Spring – WHS Poster Gala, held on Friday, April 27th from 7:15 p.m. to 8:45 p.m.
Wound Care Advantage Launches Advanced Discovery Research Alliance
SIERRA MADRE, Calif.–(BUSINESS WIRE)–Apr 24, 2018–Wound Care Advantage (WCA), in collaboration with internationally renowned podiatric surgeon Dr. David G. Armstrong, the Southwestern Academic Limb Salvage Alliance (SALSA) and Gen1 Research, has launched Advanced Discovery, a new investigative research alliance dedicated to revolutionizing patient care through evidence-based science. Advanced Discovery will offer access to an international network of advanced wound and hyperbaric treatment centers, laying the groundwork for meaningful investigative research. In partnership with approved manufacturers and research facilitators, the Alliance will evaluate a variety of diagnostic and treatment modalities with a unified goal of positively impacting wound healing outcomes while reducing the cost of treatment. Gen1 will provide extensive administrative support and infrastructure for the studies.
Chronic wounds affect approximately 5.7 million patients in the U.S., costing the healthcare system more than $20 billion annually. 1 This burden is growing, due to a rapid increase in diabetes and vascular disease, an aging population, and rising healthcare costs. In fact, the cost of diabetic foot ulcers is greater than that of the five most costly forms of cancer 2 and diabetic foot ulcer patients are twice as costly to U.S. Medicare as those with diabetes alone … read more
Organogenesis Showcases New Product Offerings
and Research at SAWC Spring 2018
CANTON, Mass. and CHARLOTTE, N.C., April 24, 2018 /PRNewswire/ — The latest advanced wound care research and product offerings from Organogenesis Inc. – including the recently-launched PuraPly® Antimicrobial 1.6 cm small size – will be showcased during the nation’s largest interdisciplinary wound care forum, the Symposium on Advanced Wound Care Spring | Wound Healing Society (SAWC Spring | WHS) meeting, held April 25-29 in Charlotte, NC.
Organogenesis Inc., a leading regenerative medicine company focused on the development, manufacture and commercialization of product solutions for the advanced wound care, surgical and sports medicine markets, will feature its full suite of advanced wound care and surgical biologic product offerings at exhibit booth #419.
Scientific presentations will feature several products within the Organogenesis portfolio, and exhibit booth attendees will have the chance to learn more about the company’s comprehensive wound care portfolio designed to empower personalized healing for a wide variety of wound types across the wound care continuum.
Organogenesis is also a proud sponsor of the Thursday, April 26 Lunch Symposium, “Understanding the Latest Evidence: A Fresh Look at the Use of Skin Substitutes Across the Wound Care Continuum,” featuring speakers Daniel Kapp, MD; George Koullias, MD; and Katie C. Mowry, PhD.
Medline to Highlight Holistic Approach to Wound Care
and Skin Health at Symposium on Advanced Wound Care
NORTHFIELD, Ill.–(BUSINESS WIRE)–Medline today announced the launch of a new Skin Champion Program at this year’s Symposium on Advanced Wound Care (SAWC) in Charlotte, April 25-29. With more than 6.5 million people in the U.S. suffering from chronic wounds, the Medline Skin Champion Program will provide new approaches to those who battle and treat non-healing wounds across the continuum of care and provide easy-to-use education and tools for wound care clinicians and leaders.
Medline also will host a live studio news show from SAWC, featuring nearly 20 key advanced wound care and tissue regeneration opinion leaders who will provide clinical expertise and insight to the more than 2,000 physicians and nurses visiting the conference.
“Wound care has become increasingly complex with the rise in patient acuity and ongoing reimbursement challenges,” says Jonathan Primer, group president, advanced wound care, Medline. “For clinicians, this new landscape is creating greater demand for education and fueling a deeper desire to discover new ways to improve clinical practice and patient care.”
Located at booth 1025, Medline will unveil the new Skin Champion Program developed by certified WOC nurses and designed to provide education and tools that help train their teams to make skin health second nature. Dozens of pre-built educational modules address four common issues impacting wound care, including pressure injuries, skin care, wound etiologies and special populations.
MiMedx to Highlight Efficacy of Its Allografts
in Presentations at SAWC Spring Conference
Breakfast Symposium to Underscore Statistically Significant Evidence of EpiFix® in Healing DFUs and VLUs
MARIETTA, Ga., April 23, 2018 /PRNewswire/ — MiMedx Group, Inc. (NASDAQ: MDXG), a leading developer and marketer of regenerative and therapeutic biologics, today announced the Company’s participation in the 2018 Symposium on Advanced Wound Care Spring | Wound Healing Society (SAWC Spring/WHS) meeting being held April 25-29, 2018 in Charlotte, North Carolina.
During the five-day conference, the clinical and cost-effective healing results of the Company’s allografts will be highlighted in a Breakfast Symposium, two dinner presentations, five clinical poster presentations, and in-booth education sessions. MiMedx will be exhibiting in booth #830 from April 26, 2018 through April 28, 2018.
Now in its 31st year, the SAWC Spring/WHS conference is the leading national wound healing forum connecting the foremost experts with your entire wound care team to improve patient outcomes through education. No other wound care conference offers the level of education, advanced state-of-the-art clinical reviews and emerging research findings.
MiMedx will sponsor a Breakfast Symposium entitled “EpiFix® – First and Only Amnion/Chorion Allograft with Statistically Significant Level I Evidence for healing in DFUs and VLUs” on Friday morning, April 27, 2018 from 7:30 am to 9:00 am. The Company will also host two dinner presentations on Thursday, April 26th and Friday, April 27th entitled “Using MiMedx Placental Tissues in the Lower Extremity“, which will review the use of various products in wound and surgical applications.
Hands-on product demonstrations will be provided by expert physicians during booth hours on April 26th and April 27thin the MiMedx booth.
The poster presentations will report on the clinical effectiveness of MiMedx EpiFix dHACM (dehydrated Human Amnion/Chorion Membrane) placental tissue allografts in the healing of diabetic foot ulcers, venous leg ulcers, pressure ulcers and refractory non-healing wounds, as well as the use of AmnioCord umbilical cord allografts for reducing Achilles tendon pain. These independent case studies and respective conclusions will include:
- Abstract: “EpiFix VLU Study Group: A Multicenter Randomized Controlled Trial Evaluating the Efficacy of Dehydrated Human Amnion/Chorion Membrane Allograft for the Treatment of Venous Leg Ulcers”
Authors: Christian Bianchi, MD, FACS; Shawn Cazzell, DPM, FACFAS; Dean Vayser, DPM, FACFAS; Alexander M. Reyzelman, DPM, FACFAS; Hasan Dosluoglu, MD, FACS; Gregory Tovmassian, DPM - Abstract: “EpiFix DFU Study Group: A Confirmatory Study on the Efficacy of Dehydrated Human Amnion/Chorion Membrane (dHACM) Allograft in the Management of Diabetic Foot Ulcers: A Prospective, Multicenter, Randomized, Controlled Study”
Authors: William Tettelbach, MD; Shawn Cazzell, DPM; Alexander M. Reyzelman, DPM; Felix Sigal, DPM; Joseph M. Caporusso, DPM; Patrick S. Agnew, DPM - Abstract: “The Application of Dehydrated Human Amnion Chorion Membrane dHACM Allografts to Expedite Healing in Patients with Six Major Types of Refractory Non-Healing Wounds, 157 Patients”
Authors: Aamir Mahmood, DPM; Justin Goldsmith, DPM; Anna Tien, DPM; Mike Czurylo, DPM; Laith Shaman, DPM; Kelda Beachy, DPM; Neal Patel, DPM; Shayan Alamgir, DPM; Matthew Garoufalis, DPM, FASPS, FACFAOM - Abstract: “Use of Umbilical Cord Allograft for Pain Reduction in Achilles Tendon Pathologies: A Case”
Authors: Brandon Brooks, DPM; Kevin Pham, DPM; Bradley Brooks, DO; Brady Brooks, MS-1; James Henry, MS-1; Terria Madison, DPM - Abstract: “Safety and Efficacy of Weekly Application of Dehydrated Human Amnion/Chorion Membrane in the Treatment of Pressure Ulcers: a Case Series”
Authors: Chi Chi Berhane, MD, MBA; Kimberly Brantley, BSN, RN, CRRN; Sandra Williams, NP-C, APN, WCN; Erica Sutton, MA; Carlyn Kappy, RD, LD, CCRP
“For more than 30 years, SAWC and WHS have been dedicated to continuous advancements in wound care and have worked tirelessly toward their goal of decreasing the number and severity of wounds of all types.” said Parker H. “Pete” Petit, MiMedx Chairman and CEO. “We are honored to be a part of this year’s meeting, and MiMedx will continue to work with SAWC and other organizations to raise the level of scientific and clinical expertise and the professional processes within the wound care sector of healthcare.”
Bill Taylor, President and COO of MiMedx, added, “We are pleased that our EpiFix, AmnioCord and AmnioFill allografts will be so prominently demonstrated at this year’s SAWC. We look forward to participating in the conference and highlighting the significant investments made in the science and clinical study of placental technology.”
About MiMedx
MiMedx® is a leading biopharmaceutical company developing and marketing regenerative and therapeutic biologics utilizing human placental tissue allografts with patent-protected processes for multiple sectors of healthcare. “Innovations in Regenerative Medicine” is the framework behind the Company’s mission to give physicians products and tissues to help the body heal itself. The Company processes the human placental tissue utilizing its proprietary PURION® Process methodology, among other processes, to produce safe and effective allografts by employing aseptic processing techniques in addition to terminal sterilization. MiMedx has supplied over 1 million allografts to date for application in the Wound Care, Burn, Surgical, Orthopedic, Spine, Sports Medicine, Ophthalmic and Dental sectors of healthcare. For additional information, please visit www.mimedx.com.
B. Braun to Introduce 1-Ounce Prontosan® Wound Gel X at SAWC
BETHLEHEM, Pa., April 19, 2018 (GLOBE NEWSWIRE) — B. Braun Medical Inc. will introduce a patient-friendly 1-ounce tube of Prontosan®Wound Gel X at the 2018 Symposium of Advanced Wound Care, which is being held April 26-28 in Charlotte, North Carolina.
A viscous wound gel with both betaine and polyhexanide to resist microbial colonization within the dressing, Gel X softens necrotic tissue, facilitating autolytic debridement. Clear and virtually odorless, Gel X is designed to cleanse and moisten wound beds and is an effective microbial barrier. Betaine, a surfactant, promotes removal of dirt and debris, aids in the removal of wound coating, and helps prevent recontamination.1 The preservative polyhexanide inhibits the growth of micro-organisms.
Gel X is for use on diabetic foot, leg, and pressure ulcers, first and second degree burns, partial and full thickness wounds, large surface area wounds, and surgical incisions. It is now available in 1-ounce and 250-milligram tubes.
“Gel X creates a gentle healing experience and a favorable wound healing environment,” said Mike Kelly, Director of Marketing for Infection Control Product at B. Braun Medical. “It is compatible with many secondary dressings and helps eliminate needless dressing changes. The 1-ounce tube is convenient for at-home patient care and healing, and the smaller Gel X tube offers patients an economical size for personal care, as compared to the much larger 250-milligram tube that was designed for healthcare facilities.”
B. Braun’s ostomy product line, which includes base plates, pouches, and the Flexima® High Output System with Flow Collector, also will be showcased at SAWC at booth #746. The B. Braun Medical-sponsored initiative, myosto™, will be featured at the booth. Through the website myosto-mylife.com, ostomates, caregivers and clinicians can find educational information, support, and a link to request product samples.
About B. Braun
B. Braun Medical Inc., a leader in infusion therapy and pain management, develops, manufactures, and markets innovative medical products and services to the healthcare industry. Other key product areas include ostomy and wound care, dialysis, nutrition, pharmacy admixture and compounding. The company is committed to eliminating preventable treatment errors and enhancing patient, clinician and environmental safety. B. Braun Medical is headquartered in Bethlehem, Pa., and is part of the B. Braun Group of Companies in the U.S., which includes B. Braun Interventional Systems, Aesculap® and CAPS®.
Globally, the B. Braun Group of Companies employs more than 61,000 employees in more than 64 countries. Guided by its Sharing Expertise®philosophy, B. Braun continuously exchanges knowledge with customers, partners and clinicians to address the critical issues of improving care and lowering costs. To learn more about B. Braun Medical, visit www.BBraunUSA.com.
1 Bradbury S, Fletcher J. Prontosan Made Easy. Wounds Int. 2011; 2(2): 1-6.
Contact:
Jason Ford
B. Braun Medical Inc.
610.997.4722
jason.ford@bbraunusa.com
Press Release from Nasdaq GlobeNewswire
HMP’s Why Wound Care?™
Initiative Launches Web Portal for Medical Students
MALVERN, PA. (PRWEB) APRIL 09, 2018
In an effort to further prepare medical professionals about the proper management and treatment of patients with wounds, HMP, a leading healthcare event and education company, today announced the launch of a new web portal designed exclusively for medical students as part of its Why Wound Care? (WWC) initiative.
Created in 2015, the Why Wound Care? initiative informs medical and nursing students, recent graduates, and faculty about rewarding careers in wound care while offering educational resources to supplement current academic curricula where wound care education may be limited.
With the development of the new portal, medical students and faculty now have access to the following complimentary, evidence-based wound care resources:
-Sixteen video modules covering the fundamentals of wound care, including
Burns, Surgical Wound Closure, Wound Epidemiology, Pressure Injuries, Diabetic Foot Ulcers, Atypical Wounds, Wound Infections, and more;
-Downloadable PDF’s of all 25 chapters of Chronic Wound Care: The Essentials e-Book, the “gold standard” of wound care textbooks;
-Information about other continuing education resources, including wound care conferences and medical journals.
“The launch of the medical student portal greatly expands the scope of our Why Wound Care? initiative,” said Peter Norris, executive vice president, HMP. “Three years ago, we introduced WWC to nursing students. Since then, the portal has become a go-to source of free wound care content with thousands of visitors accessing modules and other materials. With the addition of the medical student portal, we are able to extend the reach of wound care education to new audiences to ultimately help improve the care of patients who suffer from acute or chronic wounds.”
The WWC medical student advisory board—comprised of 16 world-class wound care physician specialists affiliated with some of the top medical schools in the U.S., including Harvard, Penn, Stanford, Columbia, Miami, and Georgetown—contributed to the development of the materials.
“This project represents our opportunity to give back to future generations of medical students,” said Robert Kirsner, MD, PhD, Chair and Harvey Blank Professor, Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, and chair, WWC medical student advisory board. “For more than 30 years, I’ve had the good fortune to practice wound care, conduct research, and educate students, residents, and fellows as a faculty member at the University of Miami. Wound care will only receive increased attention in clinical practice due to the aging population and the rising incidences of Diabetes Mellitus and obesity. Medical students now have an excellent resource by which to learn more about wound care to either better educate themselves about evidence-based treatment and management of chronic wounds, or to consider becoming a practicing wound care physician.”
To learn more about Why Wound Care?, or to take advantage of these free resources, please visit whywoundcare.com.
About HMP
HMP is the force behind Healthcare Made Practical—and is a multichannel leader in healthcare events and education, with a mission to improve patient care. The company produces accredited medical education events and clinically relevant, evidence-based content for the global healthcare community across a range of therapeutic areas. Its brands include Consultant360, the year-round, award-winning platform relied upon by primary care providers and other specialists; Psych Congress, the largest independent mental health meeting in the U.S.; EMS World Expo, North America’s largest EMT and paramedic event; and the Symposium on Advanced Wound Care (SAWC), the largest wound care meeting in the world. For more information, visit hmpglobal.com.
Original release from PRWeb
New telemed add-on manages risk, aims to avert lawsuits in wound care
A new technology solution hopes to help long-term care professionals avoid potential lawsuits, long before a wound even heals.
Illinois-based Telemedicine Solutions LLC has announced the launch of its WoundRounds Defender, which is a feature set of its telemedicine platform for treating wounds. The add-on helps those in skilled nursing to automate documentation, from admission to discharge, as well as photographing wounds of high-risk patients.
This can come especially in handy, the company notes, for documenting complex wounds, pressure ulcers and infections, which can pose the greatest potential liability to long-term care operators.
“WoundRounds Defender gives healthcare providers legal peace of mind so they can focus on delivering the best care and patient outcomes,” Mike Diamond, CEO of Telemedicine Solutions, said in a release. “The launch of WoundRounds Defender further positions WoundRounds as the leading wound management and risk prevention solution for healthcare providers.”
Corstrata Foot Ulcer Prevention Solution
Named Among Semifinalists For T1D Exchange 2018 Diabetes Innovation Challenge
Corstrata provides new tech empowered diabetic foot ulcer prevention to improve patient lives.
CORSTRATA, a provider of digital healthcare IT solutions and services for wound management, announced today that the company’s Diabetic Foot Ulcer & Amputation Prevention solution was named as a semifinalist in the T1D Exchange 2018 Diabetes Innovation Challenge. Corstrata was one of 30 semifinalists chosen from academic researchers and early stage companies from around the world that submitted entries for solutions to support advanced diabetes care. T1D Exchange is a nonprofit research and collaboration organization dedicated to accelerating novel treatments to improve the care of people living with type 1 diabetes (T1D).
Diabetic foot ulcers (DFUs) are a common, limb-threatening, and expensive complication of diabetes. Of the 29M people with diabetes in the U.S., 1.7M suffer with one or more DFUs annually and 80K of these diabetics ultimately require an amputation. The risk of death at 5 years for DFU patients is 2.5 times as high as the risk for a patient with diabetes without a foot ulcer.
Corstrata offers a technology-enabled care management solution for diabetics at risk for formation of costly diabetic foot ulcers and related amputations. Using a “smart” mat to detect potential ulcers, Corstrata’s wound specialists engage with the patient through a mobile engagement app for timely intervention and prevention of ulcers. Corstrata’s Diabetic Foot Ulcer & Amputation Prevention Program serves as an outsourced end-to-end technology-enabled solution for value-based organizations and payers … read more
ARANZ Medical Launches Two New Silhouette Image Capture Products
CHRISTCHURCH, New Zealand, March 28, 2018 /PRNewswire/ — Two new smart-phone based imaging products, SilhouetteLite+ and SilhouetteLite, recently launched by ARANZ Medical Limited have been adopted by Promore Pharma AB (Solna, Sweden) for a multi-site clinical trial, and by New Zealand’s Capital and Coast District Health Board for their community-based nurses.
Easy-to-use, SilhouetteLite+ and SilhouetteLite apps are highly portable, yet deliver robust accuracy and image consistency. The measurement and documentation data collected is securely synchronized in ARANZ Medical’s proprietary, cloud database, SilhouetteCentral, for ease of analysis and reporting.
Promore Pharma purchased 20 SilhouetteLite+ units for their two-year venous leg ulcer study. Chief Scientific Officer, Dr. Margit Mahlapuu, stated, “The SilhouetteLite+ app and documentation system is easy for us to use across multiple locations. With it, we can ensure that the data we collect at each site is not only consistent and secure, but also easily accessed for analysis and reporting.”
Wound Care Education Institute Launches Pioneering Nutrition Wound Care Course
BROOKFIELD, Wis., March 20, 2018 /PRNewswire/ — To help Registered Dietitians (RD) and Registered Dietitian Nutritionists (RDN) prepare for board certification in wound care, Wound Care Education Institute (WCEI) has launched a tailored Skin and Wound Management course suited for the RD and RDN. The course reinforces knowledge with real-world, practical skin and wound management training while preparing the RD and RDN for the Nutrition Wound Care Certified (NWCC) certification.
“The course addresses the need for nutritionists on the wound care team and can help an RD or RDN become a nutrition wound care expert,” said WCEI Co-Founder/Clinical Instructor Nancy Morgan, RN, BSN, MBA, WOC, WCC, DWC, OMS.
Poor nutrition can have a substantial negative effect on the ability to build new tissue and defend against infection. A patient’s nutritional needs can change in the presence of wounds reinforcing the body’s requirement for a proper mix of both macro and micronutrients during wound healing. Patients with chronic and non-healing wounds often have special nutrient needs as well as other healing obstacles at play … read more
Researchers are Creating an App to Track and Analyze Chronic Wounds
With a $1.6 million award from the National Institutes of Health, an interdisciplinary research team at Worcester Polytechnic Institute is developing a smartphone app that could lower healthcare costs and improve care.
An interdisciplinary team of researchers at Worcester Polytechnic Institute (WPI) has received a four-year, $1.6 million award from the National Institutes of Health (NIH) to develop a smartphone app that will allow patients and their caregivers to track and assess chronic wounds, helping lower costs associated with frequent doctor and hospital visits, and catching serious complications before they lead to expensive hospitalizations and life-altering amputations.
Emmanuel Agu, associate professor of computer science and coordinator of WPI’s Mobile Graphics Research Group, is the principal investigator for the project. Co-principal investigators include Diane Strong, a professor in WPI’s Foisie Business School, Bengisu Tulu, an associate professor in the Foisie Business School, and Peder Pedersen, a retired professor of electrical and computer engineering.
The app, which is in the early development stage, is being designed to be used by patients and caregivers, including visiting nurses, who need to regularly check the status of potentially dangerous wounds in the home environment. It will make patients more involved in their own care, cut down on unnecessary doctor visits, and issue alerts when an emergency trip to the doctor or hospital is necessary, Agu said.
A 2017 study found that chronic, nonhealing wounds affect 5.7 million people in the United States, or about 2 percent of the population, at an annual cost of $20 billion. The cost of just transporting chronic wound patients to medical visits is estimated at about $200 million per year. “This is a big problem,” said Agu. “Wounds, wound management, and amputations have a huge cost, both financially and physically, for the people who suffer from them, as well as for their families. I like to work on real problems that make a difference for people. Much of my research is in imaging and computer graphics. Wound management is a problem that imaging technology can help with.”
Patients or their caregivers will use the app to photograph chronic wounds with a smartphone. Machine learning algorithms built into the app will measure wound assessment metrics, including size, depth, and color, which indicate how the wound healing is progressing. The algorithms will compare the readings over time to determine if the wound is shrinking or expanding, or if there are other changes, like darkening tissue, that could indicate a growing infection or other complication. The app will also compute a healing score that tells the patient whether the wound is getting better, is unchanged, or is worsening. Finally, the app will suggest one of three actions: stay the course, consult a wound specialist for advice regarding treatment, or seek immediate care.
The new wound app is an evolution of work Agu and his research team completed for Sugar, an app designed to help people with diabetes track and manage their weight and blood sugar levels, and also photograph and assess the status of any chronic foot ulcers. In the current research, Agu will build on the wound assessment component of Sugar, which was developed with a $1.2 million grant awarded in 2011 by the National Science Foundation (NSF). And while Sugar focused exclusively on diabetic wounds on the feet and legs, the new app is expected to one day be expanded to assess a broader array of chronic wounds, including arterial, venous, and pressure ulcers, also known as bed sores.
Agu, Pedersen, and Clifford Lindsay, assistant professor of radiology at University of Massachusetts Medical School, will lead an effort to address one of the key technical challenges of the new project: processing imperfect smartphone photos taken by amateurs. Agu said his team understands that patients are likely to take photos from too far away or too close up, from an angle, or under poor lighting. He said shadows are particularly problematic for wound analysis, because they affect how the computer vision algorithm perceives the wound’s colors and depth.
To address this problem, the team will develop algorithms similar to those used in facial recognition software that can transform each image so it appears as if it was taken straight on, at the proper distance, and under ideal lighting conditions. They will use techniques from an area of computer science called computational photography. “Computational photography has been applied to natural images, like landscapes, but not in the medical domain,” Agu said. “This is cutting-edge research that we believe will produce a good solution to this problem.”
In addition to fixing the images, the research team will need to train their algorithms to properly assess and interpret them, work that will draw on the decision support expertise of Strong and Tulu. The team will expose their algorithms to hundreds of images of actual wounds taken, with patient consent, by Lindsay. Raymond Dunn ‘78, chief of plastic and reconstructive surgery at UMass Memorial, is a consultant to the project.
Agu and the team will feed the photos, along with wound care specialists’ expert assessments of the wounds and what kind of treatments they require, into the machine learning system so the app will “learn” how to analyze the wounds and calculate what care advice to give. Agu’s team will also test the algorithms on realistic simulated wounds they create with special 3D printers.
“This won’t replace doctor visits entirely, but it will augment those visits,” said Agu. “Patients or caregivers can check in anytime they want using this app and get more feedback than they do with occasional doctor visits. If people self-monitor, they are more likely to change their behavior, keep a closer eye on their wounds, and take the proper care those dangerous wounds need.”
The wound app is being developed on the Android platform, but Agu expects it ultimately to be adapted for the iPhone platform as well.
About Worcester Polytechnic Institute
Founded in 1865 in Worcester, Mass., WPI is one of the nation’s first engineering and technology universities. Its 14 academic departments offer more than 50 undergraduate and graduate degree programs in science, engineering, technology, business, the social sciences, and the humanities and arts, leading to bachelor’s, master’s and doctoral degrees. WPI’s talented faculty work with students on interdisciplinary research that seeks solutions to important and socially relevant problems in fields as diverse as the life sciences and bioengineering, energy, information security, materials processing, and robotics. Students also have the opportunity to make a difference to communities and organizations around the world through the university’s innovative Global Projects Program. There are more than 40 WPI project centers throughout the Americas, Africa, Asia-Pacific, and Europe.
Contact:
Alison Duffy, Director of Public Relations
Worcester Polytechnic Institute
Worcester, Massachusetts
508-340-5040, amduffy(at)wpi(dot)edu
For the original version on PRWeb visit: http://www.prweb.com/releases/2018/02/prweb15232174.htm
Wound Care Advantage Introduces the First Referral Source Algorithm in the …
SIERRA MADRE, Calif.–(BUSINESS WIRE)–The prevalence of chronic wounds in the U.S. now impacts 6.5* million people, similar to the number of individuals diagnosed with heart failure. To help its partners increase awareness of their wound care programs in local communities, Wound Care Advantage (WCA) has launched FlightPlan™, the industry’s first referral source algorithm. FlightPlan makes it easy to identify local physicians treating patients who may be in need of advanced wound treatment.
“We look for smart ways to develop and integrate technology into all areas of our organization,” said Nick Keezer, CTO of Wound Care Advantage. “FlightPlan was designed around three main objectives: to identify providers or physicians with patients who may need specialized care; to reduce the time it takes for a wound program to connect with the local community; and to better track and manage community education efforts.”
Finding local patients that need wound care, when they need it, can be a major challenge for wound center management … read more
Stratatech Begins Test of Engineered Skin for Diabetic Foot Ulcers
Stratatech is moving forward with tests of a genetically engineered human skin that could heal the sores and wounds many diabetic patients get on their feet.
Madison, WI-based Stratatech, a subsidiary of U.K.-based Mallinckrodt (NYSE: MNK), said Wednesday that it had enrolled the first patient in a study of the skin tissue, called ExpressGraft-C9T1.
Stratatech said the ExpressGraft skin tissue builds on some of the same technologies the company pioneered in developing StrataGraft, its flagship skin replacement product. StrataGraft is cell-based skin tissue designed to coax the bodies of burn patients into regenerating skin … read more
Ancor Announces Joint Venture with Advanced Tissue
Rock, Arkansas-based Advanced Tissue, the leader in providing third-party reimbursable wound-care products in the United States.
Advanced Tissue, founded in 2000, provides advanced surgical dressings based on physician orders, in single-use unit-dose packaging, to patients in both the home as well as in long-term-care facilities. All physician-ordered wound-care supplies are customized per patient, per dressing change. Advanced Tissue simplifies and personalizes the approach to home-based dressing changes by removing the guesswork for the patient with regard to the procurement, frequency and application of the products that have been selected by their physician. This contributes to an increased positive outcome for patients that are compliant with wound-care treatments.
Tim McKibben, Ancor Managing Partner, said, “We are thrilled to partner with Advanced Tissue.” He continued, “The Advanced Tissue team has done a tremendous job building a strong and efficiently run company, and we are excited to come alongside and assist in growing Advanced Tissue to the next level. The current management team and ownership will stay in place and continue in their efforts of achieving market share in this constantly changing and growing segment of healthcare.”
Grant Funds Development of Wound Healing Glove
A group of researchers from the University of Texas at Arlington received a $227 000 grant from the federal Medical Technology Enterprise Consortium to develop the REHEAL glove. This bioengineered glove is a flexible, polymeric wound dressing made of a transparent silicone for the treatment of hand trauma. The innovative product can provide negative pressure would therapy as well as deliver therapeutics such as topical gels and irrigation. In addition, the glove allows for greater hand mobilization in the earlier phases of wound healing, which in turn accelerates rehabilitation. Previous studies have demonstrated the safety and feasibility of the product for improving patient care.
Visit https://www.eurekalert.org/pub_releases/2018-01/uota-umb013118.php for more information.
(from todayswoundclinic.com)
SANUWAVE Announces Exhibition at SAWC
SUWANEE, Ga., Jan. 19, 2018 (GLOBE NEWSWIRE) — SANUWAVE Health, Inc. (OTCQB:SNWV) is pleased to announce that the company will exhibit, SAWC (Symposium on Advanced Wound Care) in Charlotte, NC on April 25 – 29, 2018. This will be SANUWAVE’s first opportunity to present their flagship wound care device, the dermaPACE System, to the U.S. market since receiving US FDA clearance in December, allowing the Company to market the device in the US.
“We are excited to be back presenting at SAWC spring in Charlotte, our first time as an exhibitor in 8 years. Since FDA clearance, the support coming from the wound care community has been overwhelmingly positive,” Stated Kevin R. Richardson, CEO and Chairman of the Board. Mr. Richardson continued, “We are glad to be making our shift from an R&D centered company to a marketing based company. This will be the start of our reemergence in the wound care space.” The Company is using this occasion to formally introduce their lead wound care product, the dermaPACE® System. This Extracorporeal Shockwave Technology … read more
NAWCO Initiates Wound Care Certification Internationally
ST. JOSEPH, Mich., Dec. 11, 2017 /PRNewswire-USNewswire/ — National Alliance of Wound Care and Ostomy® (NAWCO®) announced today, the international expansion of its WCC® (Wound Care Certified) credential. The first group of international candidates sat for the proctored WCC® board certification exam at the conclusion of the NAWCO® approved Skin and Wound Management course in Nassau, The Bahamas on December 8, 2017.
“We are honored to be chosen as the approved credential for the Public Hospitals Authority, one of the best healthcare systems in the Caribbean. We would also like to congratulate the Wound Care Education Institute® for being chosen as the preferred education provider for the system as well. This milestone represents our continued commitment to grow the number one wound care certification in the United States to areas throughout the world where the need for more qualified wound care clinicians is great. We are excited to now offer WCC® certification beyond our American boundaries”, said Cindy Broadus, Executive Director NAWCO®.
read more
Wound care company introduces new technology
Swift Medical has introduced Swift AutoDepth technology, which lets clinicians take wound depth measurements at the point of care using a smartphone camera.
PointClickCare Skin and Wound, which is powered by Swift Medical technology, will be one of the first solutions to integrate Swift AutoDepth, the company said.
“Wounds heal from the inside out, and wound depth measurement is an important indicator in determining if a wound is healing properly,” says Carlo Perez, CEO of Swift Medical. “We saw the need for a touch-free and painless way to automate wound depth measurement and delivered with AutoDepth.”
read more
Healogics, Inc. Names David Bassin as New Chief Executive Officer
JACKSONVILLE, Fla.–(BUSINESS WIRE)–Healogics, Inc., the nation’s largest provider of advanced wound care services, today announced that it has appointed David Bassin to serve as Chief Executive Officer. Bassin joined Healogics in January 2016 as Chief Financial Officer.
“David has been an instrumental member of the executive leadership team and a catalyst for change within the organization,” said John Dineen, Chairman of the Board. Dineen went on to say, “David’s extensive leadership experience within healthcare, coupled with his understanding of the Healogics business, makes him uniquely qualified for this CEO role.”

David Bassin, Chief Executive Officer
Prior to Healogics, Bassin served as Chief Financial Officer at eviCore Healthcare, Inc., MedSolutions, Inc., and inVentiv Health, Inc. Over the course of his 20-year career, he has had the opportunity to touch many areas of healthcare, including clinical services, product management, payer strategies and, with Healogics, physician practice management. “His proven track record of driving company growth through strategic initiatives, acquisitions and operational efficiencies will allow him to seamlessly transition into this role. I am delighted that he has accepted this position,” said Dineen.
“I am excited by the opportunity to lead Healogics. It is an honor; and I’m grateful to the Board of Directors for this opportunity,” said David Bassin. “We have an exceptional organization of dedicated and talented professionals. It’s our people that make Healogics a special company and the preeminent provider of wound care. Together, with our hospital partners, we are making an incredible difference in the lives of our patients every day. This is truly an exciting time to be part of Healogics and to lead the company into the future of healthcare.”
“We are incredibly fortunate to have an executive like David leading Healogics
OnCourse Learning Acquires Leading Wound Care Training Company
Wound Care Education Institute will provide expanded offerings to healthcare clinicians
BROOKFIELD, Wis., Jan. 17, 2018 /PRNewswire/ — OnCourse Learning today announced the acquisition of Wound Care Education Institute, an internationally recognized wound care education leader for healthcare professionals.
“Wound care impacts patients along nearly the entire care continuum,” said Patrick Sheahan, President and CEO of OnCourse Learning. “By educating clinicians throughout the continuum, patient care can be more positively influenced thanks to Wound Care Education Institute’s library of courses.”
WCEI was founded in 2002 in Lake Geneva, Wis., by Nancy Morgan, RN, BSN, MBA, WOC, DWC, OMS, and Donna Sardina, RN, MHA, WCC, DWC, OMS. While working as registered nurses, they noticed a trend of nurses and healthcare professionals who had an interest in wound care but limited or non-existent access to specialized wound education. Morgan and Sardina decided to effect change by forming WCEI … read more
Mölnlycke Partners With Tissue Analytics to Simplify Chronic Wound Care
WASHINGTON, Jan. 12, 2018 /PRNewswire/ –Mölnlycke, a world-leading medical solutions company, is pleased to announce a ground-breaking partnership with Tissue Analytics, a developer of a sophisticated digital wound imaging platforms. This exciting partnership brings together Mölnlycke’s outstanding expertise in wound care and Tissues Analytics’ advanced digital capabilities.
The two parties will jointly develop and commercialize innovative digital solutions for wound care practitioners including comprehensive clinical decision support tools that will significantly simplify and standardize wound assessment and treatment.
Today, practitioners are constrained by the quality of data available for wound assessment. Conditions, such as chronic wounds, burns and pressure ulcers are evaluated using only visual approximations.
read more
Role of Omega3 – Can Activate Anti-inflammatory…
LAS VEGAS, Oct. 20, 2017 (GLOBE NEWSWIRE) — Kerecis, the company using fish skin to heal human wounds and tissue damage, will present results of multiple studies of its technology at the Symposium on Advanced Wound Care (SAWC) Fall meeting to be held October 20 to 22. Kerecis is exhibiting in booth 610 at Caesars Palace Las Vegas.
One study, which is being presented by Dr. John C. Lantis of the Mount Sinai Health System, NY, starts to elucidate the potential impact of Kerecis fish skin on anti-inflammatory pathways in wound healing. A key stage to healing of the wound is inflammation; modulating this inflammation is important to getting wound bed to heal. The study compared the fatty acid content of Kerecis Omega3 Wound to human skin, human-amnion membrane and collagen matrices, and found that fish skin contains much more Omega3 fatty acids.
In addition, this was the first time a study demonstrated that the beneficial Omega3 fatty acids modulate inflammatory response in human keratinocytes. Specifically, the pivotal study showed that fatty acids from fish skin can activate anti-inflammatory pathways in adult human keratinocytes through a Specialized Pro-Resolvin (SPM) pathway.
Researchers involved in the study believe that these results shed new light on the mechanism of action of Kerecis Omega3 Wound and may in part explain why it has been shown to improve healing rates in full-thickness wounds….